Abstract
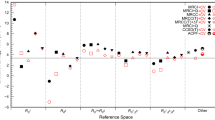
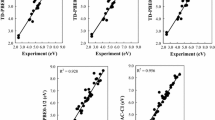
A comparison is presented of uncontracted multireference singles and doubles configuration interaction (MRCI) and internally contracted MRCI potential energy surfaces for the reaction \({\text{H}}\left( {^{2} {\text{S}}} \right) + {\text{O}}_{2} \left( {^{3} \sum\nolimits_{g}^{ - } {} } \right) \to {\text{HO}}_{2} \left( {^{2} {\text{A}}^{{\prime \prime }} } \right)\). It is found that internal contraction leads to significant differences in the reaction kinetics relative to the uncontracted calculations.






Similar content being viewed by others
References
Cobos CJ, Hippler H, Troe J (1985) J Phys Chem 89:342
Bates RW, Golden DM, Hanson RK, Bowman CT (2001) Phys Chem Chem Phys 3:2337
Hahn J, Krasnoperov L, Luther K, Troe J (2004) Phys Chem Chem Phys 6:1997
Cobos CJ, Troe J (1985) J Chem Phys 83:1010
Harding LB, Troe J, Ushakov VG (2000) Phys Chem Chem Phys 2:631
Troe J (2000) Proc Combust Inst 28:1463
Marques JMC, Varandas AJC (2001) Phys Chem Chem Phys 3:505
Harding LB, Troe J, Ushakov VG (2001) Phys Chem Chem Phys 3:2630
Lin SY, Rackham EJ, Guo H (2006) J Phys Chem A 110:1534
Sellevag SR, Georgievskii Y, Miller JA (2008) J Phys Chem 112:5085
Xu C, Xie D, Zhang DG, Lin SY, Guo H (2005) J Chem Phys 122:244035
Lischka H, Müller T, Szalay PG, Shavitt I, Pitzer RM, Shepard R (2011) WIREs Comput Mol Sci 1:191
MOLPRO, version 2010.1, a package of ab initio programs, Werner H-J, Knowles PJ, Manby FR, Schütz M, Celani P, Knizia G, Korona T, Lindh R, Mitrushenkov A, Rauhut G, Adler TB, Amos RD, Bernhardsson A, Berning A, Cooper DL, Deegan MJO, Dobbyn AJ, Eckert F, Goll E, Hampel C, Hesselmann A, Hetzer G, Hrenar T, Jansen G, Köppl C, Liu Y, Lloyd AW, Mata RA, May AJ, McNicholas SJ, Meyer W, Mura ME, Nicklass A, Palmieri P, Pflüger K, Pitzer RM, Reiher M, Shiozaka T, Stoll H, Stone AJ, Tarroni R, Thorsteinsson T, Wang M, Wolf A, see http://www.molpro.net
McLean AD, Liu B (1973) J Chem Phys 58:1066
Szalay PG, Mueller T, Gidofalvi G, Lischka H, Shepard R (2012) Chem Rev 112:108
Meyer, W (1977) In: Methods of electronic structure theory. Schaefer III HF (ed), Plenum: New York, pp 413–446
Werner H-J, Reinsch E-A (1982) J. Chem Phys 76:3144
Knowles PJ, Handy NC (1984) Chem Phys Lett 111:315
Knowles PJ, Handy NC (1989) Comp Phys Commun 54:75
Werner H-J, Knowles P (1988) J Chem Phys 89:5803
Knowles P, Werner H-J (1988) Chem Phys Lett 145:514
Kendall RA, Dunning TH Jr, Harrison RJ (1992) J Chem Phys 96:6796
Szalay PG, Bartlett RJ (1993) Chem Phys Lett 143:413
Dunning TH Jr (1989) J Chem Phys 90:1007
Klippenstein SJ (1992) J Chem Phys 96:367
Georgievskii Y, Klippenstein SJ (2003) J Phys Chem A 107:9776
Langhoff SR, Davidson ER (1974) Int J Quantum Chem 8:61
Bruckner KA (1955) Phys Rev 100:36
Burke MP, Chaos M, Ju Y, Dryer FL, Klippenstein SJ (2012) Int J Chem Kinet 44:444
Burke MP, Chaos M, Dryer FL, Ju Y (2010) Combust Flame 157:618
Burke MP, Dryer FL, Ju Y (2010) Proc Combust Inst 33:905
Acknowledgments
This work was supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences, under Contract Numbers DE-AC02-06CH11357. HL was also supported by the National Science Foundation under Project No. CHE-1213263 and by the Robert A.Welch Foundation under Grant No. D-0005.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Thom Dunning and published as part of the special collection of articles celebrating his career upon his retirement.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harding, L.B., Klippenstein, S.J., Lischka, H. et al. Comparison of multireference configuration interaction potential energy surfaces for H + O2 → HO2: the effect of internal contraction. Theor Chem Acc 133, 1429 (2014). https://doi.org/10.1007/s00214-013-1429-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-013-1429-6