Abstract.
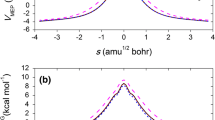
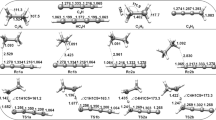
The hydrogen-abstraction reaction C2H5F+O → C2H4F+OH has been studied by a dual-level direct dynamics method. For the reaction, three reaction channels, one for α-abstraction and two for β-abstraction, have been identified. The potential-energy surface information is obtained at the MP2(full)/6-311G(d,p) and PMP2(full)/6-311G(3df,3pd) (single-point) levels. By canonical variational transition-state theory, rate constants for each reaction channel are calculated with a small-curvature tunneling correction. The total rate constant is calculated from the sum of the individual rate constants and the temperature dependence of the branching ratios is obtained over a wide range of temperatures from 300 to 5,000 K. The agreement of the rate constants with experiment is good in the experimental temperature range from 1,000 to 1,250 K. The calculated results indicate that at low temperatures α-abstraction is most likely to be the major reaction channel, while β-abstraction channels will significantly contribute to the whole reaction rate as the temperature increases.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 23 January 2002 / Accepted: 23 June 2002 / Published online: 20 September 2002
Rights and permissions
About this article
Cite this article
Liu, Jy., Li, Zs., Dai, Zw. et al. Direct ab initio dynamics calculations on the rate constants for the hydrogen-abstraction reaction of C2H5F with O (3P). Theor Chem Acc 108, 179–186 (2002). https://doi.org/10.1007/s00214-002-0371-9
Issue Date:
DOI: https://doi.org/10.1007/s00214-002-0371-9