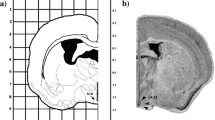
Abstract
Rationale
Nesfatin-1, derived from the protein NEFA/nucleobindin2 (NUCB2), is a newly identified peptide that acts as a potent satiety agent. It has been reported that peptides involved in the regulation of ingestive behavior are also involved in the regulation of the stress response. However, the relation between nesfatin-1 and stressor-related behaviors like anxiety and/or fear has not yet been investigated.
Objective
The effects of intracerebroventricular (ICV) injection of nesfatin-1 (0, 5, and 25 pmol/3 μl) were assessed in several paradigms that are thought to reflect anxiety and/or fear in rats.
Results
Consistent with an anxiogenic effect, nesfatin-1 dose-dependently decreased the percentage of time spent on the open arms of the elevated plus maze, increased latency to approach, and decreased consumption of a palatable snack in an anxiogenic (unfamiliar) environment. Moreover, ICV nesfatin-1 increased the fear-potentiated startle response and the time spent freezing to both context and conditioned cues in a conditioned emotional response test.
Conclusions
These findings suggest that in addition to its role as a satiety peptide, nesfatin-1 may also be involved in the mediation of anxiety- and/or fear-related responses.




Similar content being viewed by others
References
Ahima RS, Flier JS (2000) Leptin. Annu Rev Physiol 62:413–437
Akana SF, Strack AM, Hanson ES, Dallman MF (1994) Regulation of activity in the hypothalamic–pituitary–adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology 135:1125–1134
Al-Damluji S, Iverson T, Thomas JM, Pendlebury DJ, Rees LH, Besser GM (1987) Food-induced cortisol secretion is mediated by central alpha-1 adrenoceptor modulation of pituitary ACTH secretion. Clin Endocrinol 26:629–636
Anisman H, Merali Z, Hayley S (2008) Neurotransmitter, peptides and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol 85:1–74
Blanchard RJ, Blanchard DC (1969) Crouching as an index of fear. J Comp Physiol Psychol 67:370–375
Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ (2007) Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 148:5088–5094
Carobrez AP, Bertoglio LJ (2005) Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29:1193–1205
Chaki S, Ogawa S, Toda Y, Funakoshi T, Okuyama S (2003) Involvement of the melanocortin MC4 receptor in stress-related behavior in rodents. Eur J Pharmacol 474:95–101
Crawley JN, Corwin RL (1994) Biological actions of cholecystokinin. Peptides 15:731–755
Cruz APM, Frei F, Graeff FG (1994) Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav 49:171–176
Dallman MF, Akana SF, Strack AM, Hanson S, Sebastian RJ (1995) The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at sites proximal to CRF neurons. Ann NY Acad Sci 771:730–742
Davis M (1990) Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmacol Ther 47:147–165
Davis M (1992) The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15:353–375
Davis M (1993) Pharmacological analysis of fear-potentiated startle. Braz J Med Biol Res 26:235–260
Davis M, Falls WA, Campeau S, Kim M (1993) Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res 58:175–198
Dhillo WS, Small CJ, Seal LJ, Kim MS, Stanley SA, Murphy KG, Ghatei MA, Bloom SR (2002) The hypothalamic melanocortin system stimulates the hypothalamo-pituitary-adrenal axis in vitro and in vivo in male rats. Neuroendocrinology 75:209–216
File SE (1992) Behavioural detection of anxiolytic action. In: Elliot JM, Heal DJ, Marsden CA (eds) Experimental approaches to anxiety and depression. Wiley, Chichester, pp 25–44
Follenius M, Brandenberger G, Hietter B (1982) Diurnal cortisol peaks and their relationships to meals. J Clin Endocrinol Metab 55:757–761
Fort P, Butaud C, Salvert D, Shimizu H, Hashimoto K, Mori M, Luppi PH (2007) The satiety molecule nesfatin-1 is co-localized with MCH in hypothalamic neurons expressing Fos during paradoxical sleep in rats. Société des Neuroscience (8e Colloque Montpellier) Abstract:B.13
Gosnell BA, Morley JE, Levine AS (1983) A comparison of the effects of corticotropin releasing factor and sauvagine on food intake. Pharmacol Biochem Behav 19:771–775
Griebel G, Rodgers RJ, Perrault G, Sanger DJ (1997) Risk assessment behaviour: evaluation of utility in the study of 5-HT-related drugs in the rat elevated plus-maze test. Pharmacol Biochem Behav 57:817–827
Hanson ES, Dallman MF (1995) Neuropeptide Y (NPY) may integrate responses of hypothalamic feeding systems and the hypothalamic–pituitary–adrenal axis. J Neuroendocrinol 7:273–279
Heinrichs SC, Menzaghi F, Pich EM, Britton KT, Koob GF (1995) The role of CRF in behavioral aspects of stress. Ann NY Acad Sci 771:92–104
Kalin NH, Takahashi LK (1990) Fear-motivated behavior induced by prior shock experience is mediated by corticotropin-releasing hormone systems. Brain Res 509:80–84
Karbonits M, Trainer PJ, Nelson ML, Howse I, Kopelman PG, Besser GM, Grossman AB, Svec F (1996) Differential stimulation of cortisol and dehydroepiandrosterone levels by food in obese and normal subjects: relation to body fat distribution. Clin Endocrinol 45:699–706
Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T (2008) Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology 149:1295–1301
Koob GF, Heinrichs SC (1999) A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res 848:141–152
Leal AM, Moreira AC (1997) Food and the circadian activity of the hypothalamic–pituitary–adrenal axis. Braz J Med Biol Res 30:1391–1405
Levine AS, Morley JE (1981) Stress-induced eating in rats. Am J Physiol 241:R72–R76
Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY (2007) The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology 148:5531–5540
Lu XY, Barsh GS, Akil H, Watson SJ (2003) Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 23:7863–7872
Marti O, Marti J, Armario A (1994) Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav 55:747–753
Merali Z, McIntosh J, Kent P, Michaud D, Anisman H (1998) Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci 18:4758–4766
Merali Z, Levac C, Anisman H (2003) Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry 54:552–565
Merali Z, Khan S, Michaud DS, Shippy SA, Anisman H (2004) Does amygdaloid corticotropin-releasing hormone (CRH) mediate anxiety-like behaviors? Dissociation of anxiogenic effects and CRH release. Eur J Neurosci 20:229–239
Moghaddam B, Bunney BS (1989) Ionic composition of microdialysis perfusing solution alters the pharmacological responsiveness and basal outflow of striatal dopamine. J Neurochem 53:652–654
Morley JE, Levine AS (1982) Corticotropin-releasing factor, grooming and ingestive behavior. Life Sci 31:1459–1464
Morley JE, Levine AS, Rowland NE (1983) Minireview. Stress induced eating. Life Sci 32:2169–2182
Oh I, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M (2006) Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443:709–712
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, New York
Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF (2004) Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology 145:3754–3762
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Piazza PV, Le Moal M (1997) Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Rev 25:359–372
Price CJ, Hoyda TD, Samson WK, Ferguson AV (2008) Nesfatin-1 influences the excitability of paraventricular nucleus neurons. J Neuroendocrinol 20:245–250
Rao TL, Kokare DM, Sarkar S, Khisti RT, Chopde CT, Subhedar N (2003) GABAergic agents prevent alpha-melanocyte stimulating hormone induced anxiety and anorexia in rats. Pharmacol Biochem Behav 76:417–423
Richard D, Lin Q, Timofeeva E (2002) The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol 440:189–197
Rodgers RJ, Dalvi A (1997) Anxiety, defense and the elevated plus-maze. Neurosci Biobehav Rev 21:801–810
Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR (1998) A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139:4428–4431
Schulkin J (2006) Angst and the amygdala. Dialogues in Clinical Neuroscience 8:407–416
Schwartz MW, Dallman MF, Woods SC (1995) Hypothalamic response to starvation: implications for the study of wasting disorders. Am J Physiol 269:R949–R957
Shiraishi I, Honma K, Honma S, Hiroshige T (1984) Ethosecretogram: relation of behavior to plasma corticosterone in freely moving rats. Am J Physiol 247:R40–R45
Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG (2006) Orexins in the regulation of the hypothalamic–pituitary–adrenal axis. Pharmacol Rev 58:46–57
Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor (CRF)-immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186
Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR (2007) Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav 91:440–448
Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ (1997) High-fat feeding alters both basal and stress-induced hypothalamic–pituitary–adrenal activity in the rat. Am J Physiol 273:E1168–E1177
Ueta Y, Ozaki Y, Saito J, Onaka T (2003) Involvement of novel feeding-related peptides in neuroendocrine response to stress. Exp Biol Med 228:1168–1174
Valles A, Marti O, Garcia A, Armario A (2000) Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am J Physiol 279:R1138–R1144
Vergoni AV, Bertolini A (2000) Role of melanocortins in the central control of feeding. Eur J Pharmacol 405:25–32
Walker DL, Davis M (2002) Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychopharmacology 164:318–328
Acknowledgements
This study was supported by funds from the Canadian Institutes of Health Research (CIHR) and the Natural Science and Engineering Research Council of Canada (NSERC). HA is a Canadian Research Chair in Neuroscience. All experiments were conducted in accordance with the current laws of Canada. None of the authors have any direct or indirect conflicts of interests of a financial nature relevant to the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merali, Z., Cayer, C., Kent, P. et al. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology 201, 115–123 (2008). https://doi.org/10.1007/s00213-008-1252-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1252-2