Abstract
Rationale
The exact behavioral nature of drug-induced reinstatement of drug seeking is still debated. As an incentive, the drug can have general facilitatory influences on appetitive behaviors. As an interoceptive stimulus, the drug can acquire discriminative properties and control behavior.
Objective
This study assessed the relative contribution of the incentive versus discriminative properties of cocaine in food-seeking reinstatement.
Methods
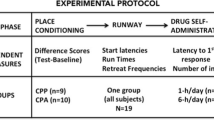
In Experiment 1, eight groups of rats were trained to press a lever for food pellets and experienced cocaine (0, 5, 10, or 15 mg/kg; i.p.), either during the operant conditioning sessions or 4 h after, in another environment without food access. In Experiment 2, to dissociate the role of the operant response per se from the consummatory response, two groups of rats experienced food consumption under cocaine (10 mg/kg; i.p.) either during operant conditioning sessions or during alternate sessions of free access to the food. Then, for both experiments, food pellets were withheld and cocaine injections ceased (extinction). The reinstating effects of noncontingent cocaine (10 mg/kg; i.p.) and food pellet delivery were assessed. Locomotor activity was recorded to probe expression of behavioral sensitization.
Results
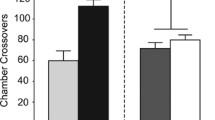
Cocaine reinstated lever pressing only in rats having previously performed the operant responses under cocaine. In contrast, food pellet delivery reinstated lever pressing independently of rats’ history with cocaine. Locomotor sensitization was evidenced for all cocaine-pre-exposed rats, dissociating sensitization from reinstatement.
Conclusions
When present during operant conditioning, the stimulus “cocaine” acquires conditioned properties which can then promote reinstatement of the extinguished behavior.






Similar content being viewed by others
References
Ahmed SH, Koob GF (1997) Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology 132:289–295
Alleweireldt AT, Weber SM, Neisewander JL (2001) Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav 69:555–560
Baker AG, Steinwald H, Bouton ME (1991) Contextual Conditioning and Reinstatement of Extinguished Instrumental Responding. Q J Exp Psychol [B] 43:199–218
Balster RL (1988) Drugs as chemical stimuli. Psychopharmacol Ser 4:1–11
Banks ML, Czoty PW, Nader MA (2007) The influence of reinforcing effects of cocaine on cocaine-induced increases in extinguished responding in cynomolgus monkeys. Psychopharmacology 192:449–456
Berridge CW (2006) Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology 31:2332–2340
Bevins RA, Palmatier MI (2004) Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev 3:143–158
Bickel WK, Kelly TH (1988) The relationship of stimulus control to the treatment of substance abuse. NIDA Res Monogr 84:122–140
Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y (2005) Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol 526:36–50
Bouton ME (2002) Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 52:976–986
Bouton ME, Westbrook RF, Corcoran KA, Maren S (2006) Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry 60:352–360
Cador M, Robbins TW, Everitt BJ (1989) Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience 30:77–86
Cador M, Taylor JR, Robbins TW (1991) Potentiation of the effects of reward-related stimuli by dopaminergic-dependent mechanisms in the nucleus accumbens. Psychopharmacology 104:377–385
Canales JJ (2005) Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem 83:93–103
Colpaert FC (1999) Drug discrimination in neurobiology. Pharmacol Biochem Behav 64:337–345
Colpaert FC, Niemegeers CJ, Janssen PA (1979) Discriminative stimulus properties of cocaine: neuropharmacological characteristics as derived from stimulus generalization experiments. Pharmacol Biochem Behav 10:535–546
Conklin CA, Tiffany ST (2002) Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97:155–167
Crombag HS, Shaham Y (2002) Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116:169–173
Davidson TL (1993) The nature and function of interoceptive signals to feed: toward integration of physiological and learning perspectives. Psychol Rev 100:640–657
De Wit H (1996) Priming Effects With Drugs and Other Reinforcers. Exp Clin Psychopharmacol 4:5–10
De Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75:134–143
Devonshire IM, Mayhew JE, Overton PG (2007) Cocaine preferentially enhances sensory processing in the upper layers of the primary sensory cortex. Neuroscience 146:841–851
Epstein DH, Preston KL (2003) The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology 168:31–41
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology 189:1–16
Goddard B, Leri F (2006) Reinstatement of conditioned reinforcing properties of cocaine-conditioned stimuli. Pharmacol Biochem Behav 83:540–546
Grakalic I, Panlilio LV, Thorndike EB, Schindler CW (2007) Differential involvement of dopamine receptors in conditioned suppression induced by cocaine. Eur J Pharmacol 573:116–123
Hunt WA, Barnett LW, Branch LG (1971) Relapse rates in addiction programs. J Clin Psychol 27:455–456
Katz JL, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168:21–30
Keiflin R, Vouillac C, Cador M (2007) Level of operant training rather than cocaine intake predicts level of reinstatement. Psychopharmacology (in press)
Ma F, Falk JL, Lau CE (1999) Within-subject variability in cocaine pharmacokinetics and pharmacodynamics after intraperitoneal compared with intravenous cocaine administration. Exp Clin Psychopharmacol 7:3–12
McMillan DE, Katz JL (2002) Continuing implications of the early evidence against the drive-reduction hypothesis of the behavioral effects of drugs. Psychopharmacology 163:251–264
Miller RR, Oberling P (1998) Analogies between occasion setting and Pavlovian conditioning. In: Schmajuk NA, Holland PC (eds) Occasion setting: Associative learning and cognition in animals. American Psychological Association, Washington, DC, pp 3–35
Myers KM, Davis M (2002) Behavioral and neural analysis of extinction. Neuron 36:567–584
Nelson A, Killcross S (2006) Amphetamine exposure enhances habit formation. J Neurosci 26:3805–3812
Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RN, Pennartz CM, Vanderschuren LJ (2007) Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacol 17:532–540
O’Brien CP (1997) A range of research-based pharmacotherapies for addiction. Science 278:66–70
Odum AL, Shahan TA (2004) D-Amphetamine reinstates behavior previously maintained by food: importance of context. Behav Pharmacol 15:513–516
Olausson P, Jentsch JD, Taylor JR (2004) Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology 173:98–104
Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR (2006) DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci 26:9196–9204
Overton DA (1978) Major theories of state-dependent learning. In: Ho BT, Richards DW III, Chute DL (eds) Drug Discrimination and State Dependent Learning. Academic, New York, pp 283–318
Overton DA (1985) Contextual stimulus effects of drugs and internal states. In: Balsam PD, Tomie A (eds) Context and Learning. Hillsdale, Erlbaum, pp 357–384
Palmatier MI, Bevins RA (2007) Facilitation by drug states does not depend on acquired excitatory strength. Behav Brain Res 176:292–301
Panlilio LV, Weiss SJ, Schindler CW (2000) Stimulus compounding enhances conditioned suppression produced by cocaine-paired stimuli. Exp Clin Psychopharmacol 8:6–13
Panlilio LV, Thorndike EB, Schindler CW (2007) A stimulus-control account of regulated drug intake in rats. Psychopharmacology 196:441–450
Paulson PE, Robinson TE (1995) Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse 19:56–65
Pitts DK, Marwah J (1989) Autonomic actions of cocaine. Can J Physiol Pharmacol 67:1168–1176
Poon J, van den Buuse M (1998) Autonomic mechanisms in the acute cardiovascular effects of cocaine in conscious rats. Eur J Pharmacol 363:147–152
Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA (2004) Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci 24:3554–3562
Reid RL (1958) The role of the reinforcer as a stimulus. Br J Psychol 49:202–209
Robbins TW, Koob GF (1978) Pipradrol enhances reinforcing properties of stimuli paired with brain stimulation. Pharmacol Biochem Behav 8:219–222
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Rutter JJ, Devilbiss DM, Waterhouse BD (2005) Effects of systemically administered cocaine on sensory responses to peri-threshold vibrissae stimulation: individual cells, ensemble activity, and animal behaviour. Eur J Neurosci 22:3205–3216
Schindler CW, Thorndike EB, Ma JD, Goldberg SR (2000) Conditioned suppression with cocaine as the unconditioned stimulus. Pharmacol Biochem Behav 65:83–89
Schoenbaum G, Setlow B (2005) Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex 15:1162–1169
Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42
Stewart J (2000) Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci 25:125–136
Stewart J (2004) Pathways to relapse: factors controlling the reinitiation of drug seeking after abstinence. Nebr Symp Motiv 50:197–234
Swartzentruber (1995) Modulatory mechanisms in pavlovian conditioning. Anim Learn Behav 23:123–143
Taylor JR, Horger BA (1999) Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology 142:31–40
Vanderschuren LJ, Everitt BJ (2005) Behavioral and neural mechanisms of compulsive drug seeking. Eur J Pharmacol 526:77–88
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
Wyvell CL, Berridge KC (2000) Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20:8122–8130
Acknowledgements
We are grateful to Anne Fayoux and Stephane Lelgouach for animal care, Pierre Gonzalez for technical support, Caroline Vouillac for logistic assistance, and Marie-Hélène Bruyères for administrative assistance. We also thank Dr. Kelly Clemens for her constructive comments on an earlier version of the manuscript.
Conflict of interest statement
The authors declare no financial conflict of interest. The present work was supported by the Centre National de la Recherche Scientifique, University of Bordeaux 1 and 2, the Mission Interministérielle de Lutte contre la Drogue & la Toxicomanie, and the “Conseil Régional d’Aquitaine.” Ronald Keiflin is supported by a doctoral fellowship from the French Ministry of Research and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keiflin, R., Isingrini, E. & Cador, M. Cocaine-induced reinstatement in rats: evidence for a critical role of cocaine stimulus properties. Psychopharmacology 197, 649–660 (2008). https://doi.org/10.1007/s00213-008-1083-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1083-1