Abstract
Rationale
Tobacco use and obesity lead to significant morbidity and mortality.
Objective
This study was conducted to investigate the factors maintaining smoking behavior in lean and obese individuals by utilizing a mouse/human cross-validation model of nicotine reward.
Methods
In humans, a cigarette choice paradigm was used to examine the relative reinforcing value of nicotine in obese and non-obese smokers. Conditioned place preference (CPP) for nicotine was assessed in mice fed standard low fat rodent chow and mice rendered obese by a high fat diet.
Results
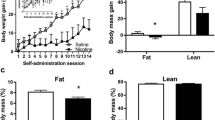
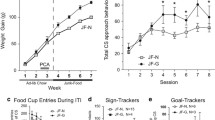
In humans, obese smokers self-administered nicotine via cigarettes significantly less often than non-obese smokers and showed attenuated hedonic effects of nicotine-containing cigarettes compared to denicotinized cigarettes. Similarly, mice exposed to a high fat diet did not exhibit nicotine CPP, relative to control mice. mRNA levels for mu-opiate and leptin receptors were also downregulated in the ventral tegmental area of these mice.
Conclusions
Together, these studies provide the first evidence for reduced nicotine reward in obese subjects and suggest that this may be mediated by dietary influences on the endogenous opioid system.


Similar content being viewed by others
References
Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM (1984) Endogenous opioids: biology and function. Annu Rev Neurosci 7:223–255
Bardo MT, Bevins RA (2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153:31–43
Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D Jr, Schwartz MW (1999) Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res 848:114–123
Berman Y, Devi L, Carr KD (1995) Effects of streptozotocin-induced diabetes on prodynorphin-derived peptides in rat brain regions. Brain Res 685:129–134
Berrendero F, Kieffer BL, Maldonado R (2002) Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci 22:10935–10940
Borrelli B, Hogen J, Bock B, Pinto B, Roberts M, Marcus B (2002) Predictors of quitting and dropout among women in a clinic-based smoking cessation program. Psychol Addict Behav 16:22–27
Brauer LH, Behm FM, Westman EC, Patel P, Rose JE (1999) Naltrexone blockade of nicotine effects in cigarette smokers. Psychopharmacology (Berl) 143:339–346
Brauer LH, Behm FM, Lane JD, Westman EC, Perkins C, Rose JE (2001) Individual differences in smoking reward from de-nicotinized cigarettes. Nicotine Tob Res 3:101–109
Calabresi P, Lacey MG, North RA (1989) Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol 98:135–140
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625–1638
Carr GD, Fibiger HC, Phillips AG (1989) Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ (eds) The neuropharmacological basis of reward. Oxford Press, New York
Carroll ME, Roth ME, Voeller RK, Nguyen PD (2000) Acquisition of oral phencyclidine self-administration in rhesus monkeys: effect of sex. Psychopharmacology (Berl) 149:401–408
Centers for Disease Control and Prevention (2002) Cigarette smoking among adults—United States 2000. MMWR 51:642–645
Corrigall WA, Herling S, Coen KM (1988) Evidence for opioid mechanisms in the behavioral effects of nicotine. Psychopharmacology (Berl) 96:29–35
Corrigall WA, Coen KM, Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653:278–284
Davenport KE, Houdi AA, Van Loon GR (1990) Nicotine protects against mu-opioid receptor antagonism by beta-funaltrexamine: evidence for nicotine-induced release of endogenous opioids in brain. Neurosci Lett 113:40–46
Dhatt RK, Gudehithlu KP, Wemlinger TA, Tejwani GA, Neff NH, Hadjiconstantinou M (1995) Preproenkephalin mRNA and methionine–enkephalin content are increased in mouse striatum after treatment with nicotine. J Neurochem 64:1878–1883
Epstein AM, King AC (2004) Naltrexone attenuates acute cigarette smoking behavior. Pharmacol Biochem Behav 77:29–37
Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH et al (2001) Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 161:1581–1586
Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ (2001) Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav 73:229–234
Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG (2003) Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964:107–115
Flegal KM, Carroll MD, Ogden CL, Johnson CL (2002) Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395:763–770
Fulton S, Woodside B, Shizgal P (2000) Modulation of brain reward circuitry by leptin. Science 287:125–128
Grunberg NE, Bowen DJ, Winders SE (1986) Effects of nicotine on body weight and food consumption in female rats. Psychopharmacology (Berl) 90:101–105
Heatherton T, Kozlowski L, Frecker R, Fagerstrom K (1991) The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict 86:1119–1127
Houdi AA, Pierzchala K, Marson L, Palkovits M, Van Loon GR (1991) Nicotine-induced alteration in Tyr–Gly–Gly and Met-enkephalin in discrete brain nuclei reflects altered enkephalin neuron activity. Peptides 12:161–166
Ise Y, Narita M, Nagase H, Suzuki T (2000) Modulation of opioidergic system on mecamylamine-precipitated nicotine-withdrawal aversion in rats. Psychopharmacology (Berl) 151:49–54
Kawada T (2004) Comparison of daily life habits and health examination data between smokers and ex-smokers suggests that ex-smokers acquire several healthy-lifestyle practices. Arch Med Res 35:329–333
Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311
Kim EM, Welch CC, Grace MK, Billington CJ, Levine AS (1996) Chronic food restriction and acute food deprivation decrease mRNA levels of opioid peptides in arcuate nucleus. Am J Physiol 270:R1019–R1024
Krishnan-Sarin S, Meandzija B, O’Malley S (2003) Naltrexone and nicotine patch smoking cessation: a preliminary study. Nicotine Tob Res 5:851–857
Lee PW, Lee YM (2003) Transcriptional regulation of mu opioid receptor gene by cAMP pathway. Mol Pharmacol 64:1410–1418
Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J et al (2004a) Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Ann Intern Med 140:426–433
Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S et al (2004b) The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics Journal 4:184–192
Malin DH, Lake JR, Carter VA, Cunningham JS, Wilson OB (1993) Naloxone precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 112:339–342
Mokdad AH, Marks JS, Stroup DF, Gerberding JL (2004) Actual causes of death in the United States, 2000. JAMA 291:1238–1245
Opitz K, Weischer ML (1988) Volitional oral intake of nicotine in tupaias: drug-induced alterations. Drug Alcohol Depend 21:99–104
Perkins KA (1989) Interactions among coronary heart disease factors. Annals Behav Med 11:3–11
Perkins KA (1992) Effects of tobacco smoking on caloric intake. Br J Addict 87:193–205
Perkins K (1993) Weight gain following smoking cessation. J Consult Clin Psychol 61:768–777
Perkins KA (1999) Nicotine self administration. Nicotine Tob Res 1(Suppl):S133–S137
Perkins KA, Fonte C (2002) Effects of smoking status and smoking cessation on leptin levels. Nicotine Tob Res 4:459–466
Perkins KA, Grobe JE, Weiss D, Fonte C, Caggiula A (1996) Nicotine preference in smokers as a function of smoking abstinence. Pharmacol Biochem Behav 55:257–263
Perkins KA, Grobe JE, Caggiula A, Wilson AS, Stiller RL (1997) Acute reinforcing effects of low-dose nicotine nasal spray in humans. Pharmacol Biochem Behav 56:235–241
Perkins KA, Gerlach D, Broge M, Fonte C, Wilson A (2001) Reinforcing effects of nicotine as a function of smoking status. Exp Clin Psychopharmacol 9:243–250
Perkins KA, Broge M, Gerlach D, Sanders M, Grobe JE, Cherry C et al (2002) Acute nicotine reinforcement, but not chronic tolerance, predicts withdrawal and relapse after quitting smoking. Health Psychol 21:332–339
Perkins K, Sayette M, Conklin C, Caggiula A (2003) Placebo effects of tobacco smoking and other nicotine intake. Nicotine Tob Res 5:695–709
Pidoplichko VI, DeBiasi M, Williams JT, Dani JA (1997) Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390:401–404
Pierzchala K, Houdi AA, Van Loon GR (1987) Nicotine-induced alterations in brain regional concentrations of native and cryptic Met- and Leu-enkephalin. Peptides 8:1035–1043
Pomerleau OF, Pomerleau CS (1984) Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation. Neurosci Biobehav Rev 8:503–513
Roane DS, Iadarola MJ, Porter JR (1988) Decreased [3H]-naloxone binding and elevated dynorphin-A(1–8) content in Zucker rat brain. Physiol Behav 43:371–374
Robinson ML, Houtsmuller EJ, Moolchan ET, Pickworth WB (2000) Placebo cigarettes in smoking research. Exp Clin Psychopharmacol 8:326–332
Rose JE, Behm FM, Westman EC, Johnson M (2000) Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav 67:71–81
Rose JE, Behm FM, Ramsey C, Ritchie JC Jr (2001) Platelet monoamine oxidase, smoking cessation, and tobacco withdrawal symptoms. Nicotine Tob Res 3:383–390
Roy S, Ge BL, Loh HH, Lee NM (1992) Characterization of [3H]morphine binding to interleukin-1-activated thymocytes. J Pharmacol Exp Ther 263:451–456
Rukstalis M, Jepson C, Strasser A, Lynch K, Perkins K, Patterson F et al (in press) Naltrexone reduces the relative reinforcing value of nicotine in a cigarette smoking choice paradigm. Psychopharmacology
Salamone JD, Correa M, Mingote S, Weber SM (2003) Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther 305:1–8
Schechter MD, Calcagnetti DJ (1993) Trends in place preference conditioning with a cross-indexed bibliography; 1957–1991. Neurosci Biobehav Rev 17:21–41
Scherer G (1999) Smoking behaviour and compensation: a review of the literature. Psychopharmacology 145:1–20
Shahan TA, Bickel WK, Madden GJ, Badger GJ (1999) Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: a behavioral economic analysis. Psychopharmacology (Berl) 147:210–216
Smith SL, Harrold JA, Williams G (2002) Diet-induced obesity increases mu opioid receptor binding in specific regions of the rat brain. Brain Res 953:215–222
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117:2–10; discussion 14–20
Swan GE, Jack LM, Ward MM (1997) Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction 92:207–217
Sweeney CT, Kozlowski LT (1998) Blocking filter vents increases carbon monoxide levels from ultralight, but not light cigarettes. Pharmacol Biochem Behav 59:767–773
Tsujii S, Nakai Y, Fukata J, Koh T, Takahashi H, Usui T et al (1986) Effects of food deprivation and high fat diet on opioid receptor binding in rat brain. Neurosci Lett 72:169–173
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Uhl GR (2003) Needed: mouse/human cross validation of reinstatement/relapse models (and drug reward models) to model human substance abuse vulnerability allelic variants. Psychopharmacology (Berl) 168:42–43
Wee CC, Rigotti NA, Davis RB, Phillips RS (2001) Relationship between smoking and weight control efforts among adults in the United States. Arch Intern Med 161:546–550
Westman E, Levin E, Rose J (1992) Smoking while wearing the nicotine patch: is smoking satisfying or harmful? Clin Res 40:871A
Will MJ, Franzblau EB, Kelley AE (2003) Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci 23:2882–2888
Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A (2002) A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci 5:727–728
Acknowledgements
This work was supported in part by National Institute on Drug Abuse Grant DA-11649-01A2 (JAB) and 1F31 DA 015949 (CLW) and a Transdisciplinary Tobacco Use Research Center grant from the National Cancer Institute and National Institute on Drug Abuse P5084718 (CL). The authors acknowledge Dr. Margaret Rukstalis for her input on the nicotine choice paradigm, Dr. Neal Benowitz for his helpful feedback and for cotinine assays, Susan Kucharski and Angela Pinto for their assistance with data management, Misty Godfrey for animal husbandry, and Maryanne Foster for assistance with manuscript preparation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Blendy, J.A., Strasser, A., Walters, C.L. et al. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology 180, 306–315 (2005). https://doi.org/10.1007/s00213-005-2167-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2167-9