Abstract
Rationale
Sex differences have been reported for the impact of nicotine and nonpharmacological cues on smoking. While nonpharmacological environmental stimuli have also been shown to influence nicotine self-administration in rats, there have been no attempts to examine the impact of sex differences in the contributions of nicotine and nondrug stimuli to this behavior.
Objectives
This experiment investigated sex differences in operant responding for nicotine in rats when drug infusions were delivered either in the absence of, or in combination with, a nonpharmacological stimulus.
Methods
Initially, male and female rats acquired self-administration for nicotine alone across a range of doses (0.03, 0.06, and 0.15 mg kg−1 inf−1, freebase). After stable acquisition, nicotine infusions were combined with a weakly reinforcing, compound visual stimulus.
Results
While there was no overall effect of dose on active lever responding for nicotine in the absence of the visual stimulus, female rats responded more on the reinforced lever than males at 0.06 and 0.15 mg kg−1 inf−1 on an FR5 schedule. However, they also showed increased responding on the nonreinforced lever compared to males at the same doses. Combining nicotine infusions with the visual stimulus doubled responding compared to nicotine alone at 0.03 and 0.06, but not at 0.15 mg kg−1 inf−1: this effect was significantly greater for female rats.
Conclusions
These data highlight the prominent contribution of nonpharmacological stimuli to nicotine-reinforced behavior across a range of doses in both male and female rats. They also reveal sex differences in operant responding for nicotine under conditions where a nonpharmacological stimulus is either absent, or combined with drug delivery.
Similar content being viewed by others
Introduction
Sex differences in reactivity to abused drugs have been extensively modeled in a variety of animal species using the drug self-administration paradigm, where operant responding is reinforced by intravenous drug infusions (e.g., Cosgrove and Carroll 2003; Lynch and Carroll 2000; Campbell et al. 2002; Hu et al. 2004). Using this model, Donny et al. (2000) reported that female rats acquired nicotine (NIC) self-administration on a fixed ratio (FR) reinforcement schedule more rapidly than males, although this difference only occurred at the lowest NIC dose tested (see also Lanza et al. 2004). More notably, female rats exhibited higher breakpoints on a progressive ratio reinforcement schedule, and showed a shorter latency to the first infusion of each session under both progressive ratio and fixed ratio schedules, suggesting that females were more motivated to self-administer NIC than males. However, as noted by Donny et al., this interpretation is confounded by the fact that NIC infusions occurred concomitantly with the presentation of a weakly reinforcing, compound visual stimulus (VS; 1 s cue light onset, followed by 1 min houselight offset). Therefore, it is unclear if the sex differences in NIC self-administration observed in this experiment resulted from differential sensitivity to NIC, the VS or a combination of both.
The possibility that there may be sex differences in the contribution of NIC and non-NIC factors to smoking is supported by recent investigations. Perkins et al. (2002) reported that the subjective and reinforcing effects of smoking were less influenced by the NIC dose present in cigarettes for women than men. Compared to men, women also demonstrated reduced sensitivity to changes in NIC dose via nasal spray (Perkins 1999). Together, these data suggest that smoking may be less strongly driven by the direct effects of NIC for women than men. In contrast, female smokers appear to show greater sensitivity to nonpharmacological stimuli that are associated with cigarette use. For example, in an investigation of sex differences in the contribution of visual and olfactory smoking stimuli to smoking, blockade of olfactory stimuli significantly reduced hedonic ratings (‘like puffs,’ ‘satisfying’) as well as reinforcement (puff self-administration) for women, but not for men (Perkins et al. 2001). Collectively, these data highlight the importance of considering both nicotinic and nonpharmacological factors in explanations of sex differences in smoking, and raise the question of whether the relative impact of these two variables may also differ for NIC self-administration by male and female rats.
Within the context of animal models, the demonstrable contribution of nonpharmacological stimuli to NIC self-administration has been thoroughly investigated in male rats. Rapid, schedule-dependent acquisition of NIC self-administration was observed when drug infusions were associated with a weakly reinforcing compound visual stimulus (VS, described above) (Donny et al. 1995; Caggiula et al. 2002). However, in the absence of VS male rats exhibited lower overall levels of responding across a range of NIC doses (Caggiula et al. 2001; Donny et al. 2003). Compared to the dose-response function for NIC self-administration in the presence of the VS, the peak of the dose–effect curve for NIC alone was shifted downward and to the right, suggesting that overall reinforcement from NIC is reduced in the absence of the nonpharmacological stimulus (Donny et al. 2003). Finally, while either NIC or the VS alone produced only partial reacquisition of responding (<50% of baseline) in male rats that had undergone extinction training following stable NIC self-administration, the combined delivery of NIC and the VS resulted in rapid, complete reacquisition of lever pressing to preextinction levels (Caggiula et al. 2001). These findings substantiate the role of nonpharmacological stimuli in NIC self-administration, and prompt the hypothesis that NIC self-administration in rats results from a synergistic interaction between NIC and nondrug factors.
The evidence summarized above illustrates the impact of nonpharmacological stimuli on NIC self-administration in animals and smoking in humans, and suggests that the influence of NIC and non-NIC stimuli on smoking may differ as a function of sex. In the present study, we used the self-administration model in rats to systematically investigate sex differences in operant responding for either NIC alone, or infusions of NIC that were combined with a nonpharmacological stimulus. Initially, male and female rats were allowed to acquire self-administration for NIC without the VS across a range of doses. After stable responding was achieved the impact of subsequently combining NIC infusions with a weakly reinforcing, compound visual stimulus was assessed. This manipulation enabled us to determine whether a nonpharmacological stimulus could influence behavior after stable self-administration for NIC alone had been acquired, and allowed us to characterize sex differences in this effect.
Materials and methods
Subjects
Sprague–Dawley rats (Harlan Farms; male, 200–225 g; female 175–200 g) were individually housed in a temperature-controlled environment (21°C) on a 12-h reversed light/dark cycle (lights off at 0700 h). After habituation to the colony room (7 days), male rats were placed on a restricted diet of 20 g rat chow per day. To maintain a similar weight range for both sexes at the start of the study, female rats were given an additional week of ad libitum food before being placed on a restricted feeding schedule (20 g day−1). Animals had unlimited access to water throughout the experiment.
Apparatus
Lever-training and experimental sessions occurred in 25×31×28 cm3 operant conditioning chambers, outfitted with identical, retractable levers, a white cue light above each lever, a food pellet trough directly in-between the levers and an overhead, white chamber light located above the pellet trough. During testing animals were connected to a drug-delivery swivel system that allowed nearly unrestricted movement in the chamber. Responses on the active (reinforced) lever, inactive (nonreinforced) levers, and number of infusions were recorded using an interfaced computer software package (Med Associates, MED-PC IV). Exhaust fans within each sound-attenuating chamber produced a constant background noise (∼75 db) which masked ambient noise as well as auditory cues associated with food and drug delivery.
Training and self-administration sessions
Prior to testing, animals were trained to press the right (active) lever for 45-mg food pellets (Donny et al. 2003). Briefly, after overnight food deprivation, rats were allowed to consume 75 food pellets in a single magazine training session. The following day they were hand-shaped to press the active lever for 75 food pellets on a fixed ratio 1 schedule in a single session. Responses on the left (inactive) lever had no scheduled consequences. During magazine and shaping sessions a red chamber light, to which Sprague–Dawley rats are relatively insensitive, remained illuminated. No scheduled changes occurred in either visual or auditory stimuli during food training.
After training, rats were anesthetized with halothane, implanted with jugular catheters (Donny et al. 1999), and allowed at least 7 days to recover prior to the start of self-administration sessions. For 2 weeks following surgery rats were treated with heparin and streptokinase to help maintain catheter patency, and the antibiotic ticarcillan plus clavulanate to reduce postsurgical infections. Thereafter, catheters were flushed once daily with 0.1-ml sterile heparinized saline (30 U ml−1) on weekends, and both prior to (10 U ml−1) and after (30 U ml−1) each session on testing days. Catheter patency was determined on days 20 and 30 of the experiment by infusing a small volume of chloral hydrate through the catheter to induce a temporary loss of muscle tone. Data from animals that failed either of the chloral hydrate tests were removed from the final analysis.
Self-administration sessions began on a Monday, and were conducted on weekdays during the dark phase of the light/dark cycle. Each session lasted for 60 min, and NIC infusions were delivered intravenously, in a volume of 0.1 ml kg−1 over ∼1 s.
Experiment 1
Acquisition of NIC self-administration in the absence of nonpharmacological stimuli
Prior to the start of the experiment, rats were randomly divided into six groups (final group size=6–7/group) determined by the dose of self-administered NIC (0.03, 0.06, and 0.15 mg kg−1 inf−1, freebase) and sex of the animal. Rats were allowed to acquire NIC self-administration on an escalating fixed ratio (FR) reinforcement schedule (days 1–5, FR1; days 6–8, FR2; days 9–21, FR5). Fulfillment of the schedule requirements resulted in the delivery of an intravenous NIC infusion, followed by an unsignaled 1-min time-out period, during which responding on the active lever was recorded but not reinforced. A red chamber light remained illuminated for the duration of each test session.
Impact of a nonpharmacological stimulus on NIC self-administration
After stable self-administration for NIC alone (≤25% variation in active lever pressing on 3 consecutive days) on an FR5 schedule (phase 1, NIC-only, days 9–21), each infusion was combined with a VS presentation (phase 2, NIC+VS, days 22–28). The VS consisted of the onset of a white cue light for 1 s followed by the offset of a white chamber light for 1 min, signaling a time-out period during which responding was recorded but not reinforced (Donny et al. 2000). To further examine the contribution of the VS, it was removed for the last 4 days of the experiment (Phase 3, NIC-only, days 29–32). During this time, a red chamber light remained illuminated for the duration of each session, and lever pressing was reinforced only with NIC infusions.
Experiment 2
Sex differences in responding for a nonpharmacological stimulus in the absence of NIC
In a separate experiment, male (n=10) and female (n=9) rats were allowed to acquire responding for the VS alone, in daily 60-min sessions on an escalating FR schedule (days 1–5, FR1; days 6–8, FR2; days 9–20, FR5). These animals underwent food training as described above, but did not receive jugular catheter implant surgery and were not connected to the drug delivery system during testing.
Statistical analyses
Differences in the acquisition of NIC self-administration without the VS, and in the impact of combining NIC delivery with the VS for Experiment 1 were examined using averaged data from the last 2 days of each FR Schedule or Phase, respectively. This method was adopted to include stable data from an equal number of days at each manipulation, while avoiding potentially unstable data from the first day of a schedule change at FR2. Analysis of responding included Dose and Sex as between-subjects factors and Lever with Schedule or Phase as within-subjects factors. ANOVAs on infusions and NIC intake included Dose and Sex as between-subjects factors, and Schedule or Phase as the within-subjects factor. Identical analyses were conducted on difference scores (calculated by subtracting responding on the inactive lever from responding on the active lever), and time-out responding (active lever responses that occurred during 1 min after the delivery of a reinforcer). Main effects and interactions were subsequently tested with targeted three- and two-factor ANOVAs, followed by t-tests for paired or independent samples.
Sex differences in the impact of combining NIC with the VS were examined for infusions, NIC intake, and active and inactive lever pressing across the last 7 days of phase 1 and phase 2. Data across a wider sample of days were included in this analysis because there were no constraints imposed by having either a limited number of test days, or a change in reinforcement schedule. Sex differences in responding for the VS alone for Experiment 2 were tested using ANOVA with Day as the within-subjects variable, and Sex as the between-subjects factor. Day, rather than Schedule, was used to best characterize sex differences that emerged early in acquisition, but were significantly diminished by day 14 of the experiment. For all tests the α level for statistical significance was set to 0.05.
Results
Experiment 1
Acquisition of NIC self-administration in the absence of nonpharmacological stimuli
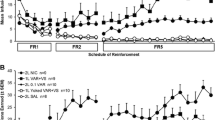
In the absence of VS, both male and female rats exhibited schedule-dependent increases in active lever responding, indicating that NIC alone was able to sustain operant responding in the face of increasing schedule demands (Fig. 1a; Schedule: p<0.001 for males and females). There was no main effect of Dose, and no Dose × Schedule interaction. A significant, schedule-dependent increase in responding on the inactive lever was observed for both male (Schedule: p<0.05) and female (Schedule: p<0.001) rats, which occurred at 0.03 mg kg−1 inf−1 for both sexes, and at 0.15 mg kg−1 inf−1 for female rats (Fig. 1b). However, responding on the inactive lever decreased across dose for both sexes (Dose: p<0.05 for males and females). For females, responding on the inactive lever was equivalent at each reinforcement schedule at 0.03 and 0.06 mg kg−1 inf−1, but significantly reduced at 0.15 mg kg−1 inf−1. For male rats, the dose-dependent decrease in inactive lever pressing occurred at a lower dose of 0.06 mg kg−1 inf−1.
Responding for nicotine alone at each dose by male and female rats. Bars represent mean (±SEM) response levels for the last 2 days on each reinforcement schedule (FR1, days 4–5; FR2, days 7–8; FR5, days 20–21). a Responding on the active (reinforced) lever. b Responding on the inactive (non-reinforced) lever. c Responding on the active lever minus responding on the inactive lever as difference scores. *Significant increase compared to FR1 for same dose (p<0.05); ^significant decrease compared to preceding dose at same schedule (p<0.05); #significantly more responding by females than males at given schedule and dose (p<0.05)
For both sexes, self-administration of NIC alone was demonstrated by significantly higher levels of responding on the active lever than the inactive lever on an FR2 and FR5 schedule, at 0.06 and 0.15 mg kg−1 inf−1 (p<0.05, compare Fig. 1a and b). There was no lever preference at the lowest NIC dose. NIC self-administration in the absence of the VS was also supported by the analysis of difference scores (Fig. 1c), which increased across schedule for both male (p<0.001) and female (p<0.001) rats. A significant Schedule × Dose interaction for female rats (p<0.05) revealed greater difference scores at 0.06 and 0.15, compared to 0.03 mg kg−1 inf−1 (FR5, p<0.05).
In the absence of the VS female rats responded more than males on both the active and the inactive levers at 0.06 (Sex: active lever, p<0.05; inactive lever, p<0.001) and 0.15 mg kg−1 inf−1 (Sex × Schedule: active lever, p<0.01; Sex: inactive lever, p<0.05). There were no sex differences in responding at the lowest NIC dose (Fig. 1a and b). Responding during the time-out period following nicotine delivery increased across schedule (p<0.0001), but there was no main effect of Dose or Sex (data not shown). A significant three-way Dose × Schedule × Sex interaction (p<0.05) confirmed that females responded more than males during the time-out on an FR5 schedule at 0.06 (mean±SEM for the last 2 days; males 9.71±2.3, females 22.83±3.5) and 0.15 mg kg−1 inf−1 (males 6.58±1.7, females 19.00±3.5). The increase in time-out responding completely accounted for the sex difference in active lever pressing at 0.15, but not at 0.06 mg kg−1 inf−1.
Impact of a nonpharmacological stimulus on NIC self-administration
NIC self-administration was immediately enhanced when drug infusions were combined with the VS (Fig. 2a–c). ANOVA results for active lever responding revealed significant effects of Phase (p<0.001 for both sexes), Dose (male, p<0.05; female, p<0.01), and Phase × Dose interactions (male, p<0.05; female, p<0.001). Responding on the active lever increased at 0.03 and 0.06 mg kg−1 inf−1, but not at 0.15 mg kg−1 inf−1 for both sexes, and responding on the inactive lever decreased significantly for females at 0.06 and 0.15 mg kg−1 inf−1 (p<0.05 vs phase 1).
Mean (±SEM) responding on the active and inactive levers for nicotine when nicotine infusions were delivered alone (NIC-only, phase 1), in combination with the VS (NIC+VS, phase 2), and after the VS was removed (NIC-only, Phase 3). *Significant increase in active lever responding during phase 2 compared to phase 1 for both males and females (p<0.01)
Combining NIC delivery with the VS resulted in a considerable dissociation between responding on the active and inactive levers, such that responding on the active lever was significantly higher than the nonreinforced lever for both sexes at each dose (p<0.01; for all comparisons of active vs inactive during phase 2 for males and females). The impact of the VS was further highlighted by the finding that removing it caused active lever pressing to decrease significantly over 4 days for both sexes at 0.03 mg kg−1 inf−1, and for females at 0.06 mg kg−1 inf−1 (p<0.05; Fig. 2a and b).
Pairing NIC with the VS caused responding during the time-out to increase for females at 0.03 mg kg−1 inf−1 (mean±SEM for the last 2 days; phase 1, 16.50±3.16; phase 2, 44.25±4.40; p<0.05). The slight increase observed for male rats at the same dose was not statistically significant (phase 1, 17.42±4.05; phase 2, 28.17±4.36). Although time-out responding remained slightly higher for female rats compared to males at each dose during phase 2, there was no statistically significant sex difference in this measure.
Infusions and NIC intake
Infusions earned in the absence of the VS remained stable across dose for both sexes, with the exception that female rats self-administered more NIC at 0.06 mg kg−1 inf−1 compared to 0.15 mg kg−1 inf−1 (p<0.05; Fig. 3a). Combining NIC with the VS produced a significant, dose-dependent elevation in infusions for each sex (Dose: male, p<0.05; female, p<0.001), and significant Phase by Dose interactions (male, p<0.05; female, p<0.001) revealed that the magnitude of this effect was greater at 0.03 compared to 0.15 mg kg−1 inf−1. Both sexes earned more infusions at each dose when NIC was delivered in combination with the VS compared to phase 1. However, female rats self-administered more NIC infusions that males at 0.06 mg kg−1 inf−1 during both phases.
Mean (±SEM) infusions (a) and nicotine intake (b) during phase 1 (NIC-only; days 20–21; circles) and phase 2 (NIC+VS; days 27–28; triangles) as a function of nicotine dose for male and female rats. #Significantly more infusions earned, or higher nicotine intake by female rats compared to males at given dose and phase; *Significant increase compared to NIC-only at same dose for both male and female rats (p<0.05)
In contrast to infusions, total NIC intake increased across dose during phase 1 for both male and female rats (0.15>0.03 mg kg−1 inf−1, p<0.05; Fig. 3b). Subsequently combining NIC with the VS caused a further increase in NIC intake at all doses (Phase: male, p<0.001; female, p<0.0001: Dose: p<0.001 for both males and females). Nevertheless, NIC intake remained highest at 0.15 mg kg−1 inf−1 for both sexes (p<0.05 compared to 0.03 mg kg−1 inf−1). As with infusions, NIC intake was greater for female rats than males at 0.06 mg kg−1 inf−1.
Analysis of sex differences revealed significant interactions between Phase and Sex at 0.06 mg kg−1 inf−1 for infusions earned (p<0.05), and NIC intake (p<0.05), indicating that the enhancement in these measures that resulted from combining NIC delivery with the VS was greater for female rats compared to males. The Phase × Sex interaction for active lever responding approached significance at the same dose (p=0.056).
Experiment 2
Sex differences in responding for a nonpharmacological stimulus in the absence of NIC
Responding on the active lever for the VS alone increased across day (Day: p<0.0001) for both sexes, although a significant Day × Sex interaction (p<0.05) indicated that female rats acquired the behavior more rapidly than males (main effect of Sex: p<0.05; Fig. 4a). Although active lever pressing and number of VS presentations earned (Fig. 4b) were significantly greater for female rats compared to males during the first 12 days of testing, responding stabilized at similar levels for both sexes towards the end of acquisition.
Discussion
These results provide evidence that nicotine (NIC) alone functions as a reinforcer, that there is a critical contribution of nonpharmacological stimuli to NIC self-administration by male and female rats, and that there are sex differences in operant responding maintained by NIC alone and by the interaction between NIC and a weakly reinforcing compound visual stimulus (VS). Below, we summarize the data in this report that support each of these statements, and discuss the main theoretical implications of these findings.
In the absence of the VS, both sexes showed schedule-dependent increases in active lever pressing, indicating that NIC alone was able to sustain self-administration across an escalating fixed ratio schedule. While response levels on the active and inactive levers were indistinguishable at 0.03 mg kg−1 inf−1 (see also Donny et al. 2003), active lever pressing was significantly higher at 0.06 and 0.15 mg kg−1 inf−1. Preferential responding on the reinforced lever can be interpreted as evidence of drug reinforcement (Woolverton and Nader 1990). Thus, our data indicate that reinforcement from NIC alone was reliably achieved at 0.06 and 0.15 mg kg−1 inf−1, but not at the lowest dose for both sexes. These observations support the widely held premise that the direct reinforcing consequences of NIC are an essential component of NIC reinforcement in animals, and smoking in humans (Donny et al. 2003; USDHHS, 1988).
The considerable impact of a nonpharmacological stimulus in NIC self-administration is demonstrated by several key findings. Compared to the high levels of operant behavior typically observed for NIC in the presence of a nonpharmacological stimulus (Corrigall and Coen 1989; Donny et al. 1998), both male and female rats responded less frequently and earned fewer infusions when NIC was delivered without the VS (present data). The concurrent delivery of NIC and the VS to rats with a history of self-administering NIC alone produced a rapid, robust enhancement in responding on the reinforced lever at lower NIC doses. Finally, withdrawing the VS caused a significant decrease in NIC self-administration for both sexes.
Subtle but significant sex differences in operant behavior were observed when NIC was self-administered in the absence of the VS. Female rats responded more on the active lever compared to males at 0.06 and 0.15 mg kg−1 inf−1. However, at these doses, they also responded more during the nonreinforced time-out period following each infusion and pressed the inactive lever more frequently than males—sex differences that have previously been reported in NIC self-administration (Donny et al. 2000). Combining NIC with the VS produced a significant increase in active responding, infusions earned, and nicotine intake at the two lowest doses tested, but the magnitude of this effect was greater for female rats than males at 0.06 mg kg−1 inf−1. Finally, female rats demonstrated more rapid acquisition of operant responding for the VS alone compared to males, although active lever pressing eventually stabilized at similar levels for both sexes.
Our initial hypothesis that nonpharmacological stimuli contribute considerably to NIC reinforcement (Caggiula et al. 2001, 2002) is substantiated in the present experiment. These data concur with prior observations that in the absence of the VS male rats self-administer substantially less NIC (Donny et al. 2003), and are the first documentation of a similar effect across a broad range of doses for female rats. The observation that female rats responded more than males on both the reinforced and nonreinforced levers at 0.06 and 0.15 mg kg−1 inf−1 suggests they are not more sensitive than males to the reinforcing properties of nicotine. Instead, the generalized increase in lever pressing by females could result from their being either more responsive to the locomotor activating properties of nicotine, or more sensitive to a combination of the direct reinforcing properties and locomotor activating effects of nicotine. Alternatively, the sex difference in responding on the inactive lever could imply that females are less able to discriminate between levers in the absence of nonpharmacological manipulations that signal drug delivery. This explanation is consistent with the results from NIC discrimination studies in humans, showing that men are better able to discriminate NIC dose than women (Perkins 1999).
Combining NIC infusions with the VS produced a dramatic enhancement in self-administration at lower doses for both male and female rats, which highlights the remarkable control over behavior exerted by a nonpharmacological stimulus delivered in combination with NIC. A fundamental question raised by these data is how does associating a relatively weak primary reinforcer, NIC, with an equally weak reinforcing VS produce such robust, dose-dependent increases in active lever pressing? One explanation is that in addition to serving as a primary reinforcer, NIC is also able to enhance the reinforcing value of other reinforcing stimuli (Donny et al. 2003). This dual-reinforcement model for NIC is similar to one proposed to explain how psychostimulants such as cocaine and pipradrol are able to increase operant responding for conditioned reinforcers (initially neutral stimuli that acquire reinforcing properties by consistent pairing with a primary reinforcer such as water) (Phillips and Fibiger 1990; Robbins et al. 1983; Robbins and Koob 1978). The application of this hypothesis to NIC is based on recent evidence showing that both response-contingent and noncontingent NIC delivery (i.e., drug delivery yoked to the lever pressing behavior of other rats or continuously infused through the session) produce equivalent, high levels of lever pressing for the VS across a range of doses (Donny et al. 2003; Chaudhri et al. 2003). This hypothesis is further supported by recent reports that acute NIC exposure elevates operant responding for a conditioned reinforcer (Olausson et al. 2004a) and chronic NIC pretreatment augments the potentiation of conditioned reinforcement by intraaccumbens amphetamine (Olausson et al. 2004b). Thus, the robust elevation in active lever pressing produced by combining NIC delivery with the VS in the present study could be attributed to NIC enhancing the reinforcing properties of the already reinforcing VS. Based on the stimulant literature (see Everitt and Wolf 2002 for review), this effect may be mediated by limbic structures like the nucleus accumbens, which plays a role in enhanced responding for conditioned reinforcers (Taylor and Horger 1999), or the basolateral amygdala, which is necessary for cue-induced reinstatement of drug-seeking behavior (see, for review, See 2002).
Associating NIC with the VS produced a larger increase in active lever pressing, infusions earned, and NIC intake for female rats compared to males at 0.06 mg kg−1 inf−1. At least two explanations could account for this sex difference. It is possible that female rats are simply more sensitive to the reinforcing properties of the VS, and data from Experiment 2 indicate that females acquire responding for the VS alone more rapidly than males. However, this observation does not rule out a second alternative that the synergistic interaction between NIC and the VS that resulted in elevated response levels may be more pronounced for female rats than their male counterpart. The interaction effect at 0.06 mg kg−1 inf−1 could result from NIC being better able to enhance the reinforcing value, and therefore incentive motivation for the VS in female rats compared to males. Further experiments are needed to test these specific hypotheses.
While it is possible that the present sex differences resulted from estrous cycle effects on NIC reinforcement, past research has failed to detect an effect of estrous cycle phase on NIC self-administration (Donny et al. 2000) by female rats. Sex differences in factors such as NIC metabolism (Schepers et al. 1993; Kyerematen et al. 1988) and NIC receptor upregulation (Koylu et al. 1997; but see also Donny et al. 2000) may partially explain the observed results. Alternatively, sex differences in limbic responses to NIC, such as the observation that systemic NIC causes greater dopamine increase in the nucleus accumbens (Corrigall et al. 1994; Wooltorton et al. 2003) for female rats compared to males (see Pogun 2001, for review), may account for these findings.
Environmental stimuli that are typically associated with smoking can induce craving and activation of brain limbic structures that presumably mediate reward (Due et al. 2002). Smoking cues (e.g., a lit cigarette in an ashtray) have also been shown to enhance measures of craving or “desire to smoke” in smokers (Perkins et al. 1994). A traditional hypothesis regarding nonpharmacological factors in smoking is that environmental stimuli become capable of eliciting craving, and thereby inducing relapse, because their continued association with NIC via smoking renders them conditioned reinforcers (see Caggiula et al. 2001 for review). In other words, the ability of such stimuli to impact smoking is caused by their prior, repeated association with nicotine. In animal models of drug self-administration, stimuli that have been associated with cocaine or NIC can induce reinstatement of lever pressing after prolonged periods of extinction in rats (Kruzich et al. 2001), and maintain operant responding within the context of a second-order reinforcement schedule (Goldberg et al. 1983), respectively. However, in the present experiment, combining NIC delivery with the VS produced a rapid elevation in self-administration in the first test session for 0.03 mg kg−1 inf−1, and a more gradual but equally robust increase at 0.06 mg kg−1 inf−1. The speed with which the impact of the VS was manifested suggests that the interaction between NIC and the VS, which results in high levels of operant responding, is nonassociative: the VS did not need to be repeatedly paired with NIC in order to impact responding. This interpretation is consistent with the hypothesis that the elevation in responding incurred by combining NIC with the VS was caused by an immediate enhancement of the reinforcing properties of the VS by NIC (Donny et al. 2003).
In summary, the present experiment revealed distinct differences in NIC self-administration when drug delivery occurred in the absence of and in combination with a weakly reinforcing visual stimulus. The considerable impact of nonpharmacological stimuli on NIC reinforcement that we observed is also a prominent factor in many human smoking studies. The close correspondence between the animal and human literature speaks to the generality of this phenomenon in nicotine-reinforced behavior. Comparisons between male and female rats revealed subtle but significant sex differences in operant responding for NIC alone, and NIC paired with the VS. Future research to test the specific hypotheses generated by these results will provide clearer insight into the mechanisms by which sex influences the complex interaction between NIC and nonpharmacological stimuli.
References
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2002) Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology 163:230–237
Campbell UC, Morgan AD, Carroll ME (2002) Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Depend 66(1):61–69
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen S, Sved AF (2003) Enhancement of reinforced operant responding by nicotine and cocaine: analysis of dose and drug-contingency. Abstr Soc Neurosci, Prog. 323.8
Corrigall WA, Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology 99:473–478
Corrigall WA, Coen KM, Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653(1–2):278–284
Cosgrove KP, Carroll ME (2003) Effects of a non-drug reinforcer, saccharin, on oral self-administration of phencyclidine in male and female rhesus monkeys. Psychopharmacology 170(1):9–16
Donny E, Caggiula AR, Knopf S, Brown C (1995) Nicotine self-administration in rats. Psychopharmacology 122:390–394
Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF (1998) Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology 136:83–90
Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE (1999) Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 147:135–142
Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S (2000) Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology 151:392–405
Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF (2003) Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology 169(1):68–76
Due DL, Huettel SA, Hall WG, Rubin DC (2002) Activation of mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry 159:954–960
Everitt BJ, Wolf ME (2002) Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22(9):3312–3320
Goldberg SR, Spealman RD, Risner ME, Henningfield JE (1983) Control of behavior by intravenous nicotine injections in laboratory animals. Pharmacol Biochem Behav 19(6):1011–1020
Hu M, Crombag HS, Robinson TE, Becker JB (2004) Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29(1):81–85
Koylu E, Demirgoren S, London ED, Pogun S (1997) Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci 61(12):PL185–PL190
Kruzich PJ, Congleton KM, See RE (2001) Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav Neurosci 115(5):1086–1092
Kyerematen GA, Owens GF, Chattopadhyay B, deBethizy JD, Vesell ES (1988) Sexual dimorphism of nicotine metabolism and ditribution in the rat. Studies in vivo and in vitro. Drug Metab Dispos 16:823–828
Lanza ST, Donny EC, Collins LM, Balster RL (2004) Analyzing the acquisition of drug self-administration using growth curves. Drug Alcohol Depend 75(1):11–21
Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology 148(2):196–200
Olausson P, Jentsch JD, Taylor JR (2004a) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology 171(2):173–178
Olausson P, Jentsch JD, Taylor JR (2004b) Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology 173(1–2):98–104
Perkins KA (1999) Nicotine discrimination in men and women. Pharmacol Biochem Behav 64(2):295–299
Perkins KA, Epstein LH, Grobe JE, Fonte C (1994) Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol Biochem Behav 47:107–112
Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S (2001) Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res 3(2):141–150
Perkins KA, Jacobs L, Sanders M, Caggiula AR (2002) Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology 163(2):194–201
Phillips AG, Fibiger HC (1990) Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behav Pharmacol 1:228–269
Pogun S (2001) Sex differences in brain and behavior: emphasis on nicotine, nitric oxide and place learning. Int J Psychophysiol 42(2):195–208
Robbins TW, Koob GF (1978) Pipradrol enhances reinforcing properties of stimuli paired with brain stimulation. Pharmacol Biochem Behav 8:219–222
Robbins TW, Watson BA, Gaskin M, Ennis C (1983) Contrasting interactions of pipradrol, d-amphetamine, cocaine, cocaine analogues, apomorphine and other drugs with conditioned reinforcement. Psychopharmacology 80:113–119
Schepers G, Rustemeier K, Walk RA, Hackenberg U (1993) Metabolism of S-nicotine in noninduced and aroclor-induced rats. Eur J Drug Metab Pharmacokinet 18(2):187–197
See RE (2002) Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71(3):517–529
Taylor JR, Horger BA (1999) Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology 142(1):31–40
USDHHS (1988) Nicotine addiction: a report of the surgeon general. US Department of Health and Human Services, Office of the Assistant Secretary for Health, Office on Smoking and Health, Rockville, MD
Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA (2003) Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci 23(8):3176–3185
Woolverton WL, Nader MA (1990) Experimental evaluation of the reinforcing effects of drugs. In: Adler MW, Cowan A (eds) Testing and evaluation of drugs of abuse, modern methods in pharmacology, vol 6. Wiley-Liss, New York, pp 165–192
Acknowledgements
This work was supported by National Institute on Drug Abuse research grants, DA-10464 and DA-12655, and by a Howard Hughes Predoctoral Research Fellowship awarded to N. Chaudhri. “Principles of laboratory animal care” (NIH No. 85-23, revised 1985) were followed throughout all experiments. The University of Pittsburgh Institutional Animal Care and Use Committee, Assurance Number A3187-01 approved this research. N. Chaudhri can be contacted at nchaudhri@egcrc.net.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaudhri, N., Caggiula, A.R., Donny, E.C. et al. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology 180, 258–266 (2005). https://doi.org/10.1007/s00213-005-2152-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2152-3