Abstract
Rationale
The nucleus accumbens receives glutamatergic and dopaminergic inputs converging onto common dendrites. Recent behavioral data demonstrated that intra-accumbens administrations of either glutamate or dopamine (DA) antagonist impair spatial memory consolidation. Thus, also based on the biochemical and molecular findings demonstrating interactions among the different receptors subtypes for glutamate and dopamine, it is conceivable that memory consolidation within this structure might be modulated by glutamate–dopamine receptor interactions.
Objectives
The purpose of this study was to examine the effects of intra-accumbens co-administrations of glutamate and DA antagonists on the consolidation of spatial information.
Methods
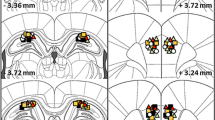
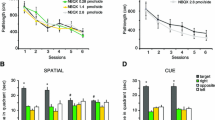
On day 1, CD1 male mice were placed in an open field containing five different objects and immediately after three sessions of habituation the animals were injected intra-accumbens with either vehicle or low doses of the N-methyl-d-aspartate (NMDA; AP-5 50 ng/side), the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA; DNQX 5 ng/side), the D1 (SCH23390 12.5 ng/side) and the D2 (sulpiride 25 ng/side) antagonists that were ineffective alone in disrupting object displacement. Separate groups were then focally injected with a combination of one of the glutamate antagonists with one of the dopamine antagonists. Twenty-four hours later, the ability of mice to discriminate object displacement was assessed.
Results
Controls and mice injected with ineffective doses of the NMDA, the AMPA, the D1 or the D2 antagonists were always able to react to the object displacement. On the contrary, the groups administered with the different combinations (AP-5 and SCH23390, AP-5 and sulpiride, DNQX and SCH23390, DNQX and sulpiride) of glutamate and dopamine antagonists did not discriminate the spatial change.
Conclusions
These results demonstrate that glutamate–dopamine receptor interactions within the accumbens are essential for the consolidation process of spatial information.







Similar content being viewed by others
References
Ariano MA, Larson ER, Noblett KL, Sibley DR, Levine MS (1997) Coexpression of striatal dopamine receptor subtypes and excitatory amino acid subunits. Synapse 26:400–414
Bardgett ME, Henry JD (1999) Locomotor activity and accumbens Fos expression driven by ventral hippocampal stimulation require D1 and D2 receptors. Neuroscience 94:59–70
Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER (1998) Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem 5:365–374
Cepeda C, Levine MS (1998) Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci 20:1–18
Cepeda C, Buchwald NA, Levine MS (1993) Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci U S A 90:9576–9580
Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME (2002) D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem 81:984–992
Coccurello R, Adriani W, Oliverio A, Mele A (2000) Effect of intra-accumbens dopamine receptor agents on reactivity to spatial and non-spatial changes in mice. Psychopharmacology 152:189–199
Das S, Grunert M, Williams L, Vincent SR (1997) NMDA and D1 receptors regulate the phosphorylation of CREB and the induction of c-fos in striatal neurons in primary culture. Synapse 25:227–233
De Leonibus E, Costantini VJA, Castellano C, Ferretti V, Oliverio A, Mele A (2003) Distinct roles of the different ionotropic glutamate receptors within the nucleus accumbens in passive-avoidance learning and memory in mice. Eur J Neurosci 18:2365–2373
Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, Kameyama K, Huganir R, Konradi C (2003) Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem 87:922–934
Floresco SB, Blaha CD, Yang CR, Phillips AG (2001) Modulation of hippocampal and amygdala-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci 21:2851–2860
Franklin BJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, San Diego
Gal G, Joel D, Gusak O, Feldon J, Weiner I (1997) The effects of electrolytic lesion to the shell subterritory of the nucleus accumbens on delayed non-matching-to-sample and four-arm baited eight-arm radial-maze tasks. Behav Neurosci 111:92–103
Greengard P, Allen PB, Nairn AC (1999) Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23:435–447
Groenewegen HJ, Wright CI, Beijer AV, Voorn P (1999) Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci 877:49–63
Hernandez PJ, Sadeghian K, Kelley AE (2002) Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nat Neurosci 5:1327–1331
Maldonado-Irizarry CS, Kelley AE (1995) Excitatory amino acid receptors within nucleus accumbens subregions differentially mediate spatial learning in the rat. Behav Pharmacol 6:527–539
McGaugh JL (2000) Memory—a century of consolidation. Science 287:248–251
Mele A, Avena M, Roullet P, De Leonibus E, Mandillo S, Sargolini F, Coccurello R, Oliverio A (2004) Nucleus accumbens and dopamine receptors in the consolidation of spatial memory. Behav Pharmacol 15:423–431
Mulder AB, Manshanden I, Vos PE, Wolterink G, van Ree JM, Lopes da Silva FH (1996) Modifications in glutamatergic transmission after dopamine depletion of the nucleus accumbens. A combined in vivo/in vitro electrophysiological study in the rat. Neuroscience 72:1009–1021
Pennartz CMA, Groenewegen HJ, Lopes de Silva FH (1994) The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioral, elettrophysiological and anatomical data. Prog Neurobiol 42:719–761
Poucet B (1989) Object exploration, habituation, and response to spatial change in rats following septal or medial frontal cortical damage. Behav Neurosci 103:1009–1016
Roullet P, Sargolini F, Oliverio A, Mele A (2001) NMDA and AMPA antagonist infusions into the ventral striatum impair different steps of spatial information processing in a non associative task in mice. J Neurosci 21:2143–2149
Sargolini F, Roullet P, Oliverio A, Mele A (1999) Effects of lesions to the glutamatergic afferents to the nucleus accumbens in the modulation of reactivity to spatial and non-spatial novelty in mice. Neuroscience 93:855–867
Sargolini F, Florian C, Oliverio A, Mele A, Roullet P (2003a) Differential involvement of NMDA and AMPA receptors within the nucleus accumbens in consolidation of information necessary for place navigation and guidance strategy of mice. Learn Mem 10:285–292
Sargolini F, Roullet P, Oliverio A, Mele A (2003b) Effects of intra-accumbens focal administrations of glutamate antagonists on object recognition memory in mice. Behav Brain Res 138:153–163
Schacter GB, Yang CR, Innis NK, Mogenson GJ (1989) The role of the hippocampal–nucleus accumbens pathway in radial-arm maze performance. Brain Res 494:339–349
Sesack SR, Pickel VM (1990) In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res 527:266–279
Sesack SR, Carr DB, Omelchenko N, Pinto A (2003) Anatomical substrate for glutamate–dopamine interactions—evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci 1003:36–52
Setlow B (1997) The nucleus accumbens and learning and memory. J Neurosci Res 49:515–521
Setlow B, McGaugh JL (1998) Sulpiride infused into the nucleus accumbens posttraining impairs memory of spatial water maze training. Behav Neurosci 112:603–610
Smith AD, Bolam JP (1990) The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci 13:259–265
Smith-Roe SL, Kelley AE (2000) Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci 22:1063–1071
Smith-Roe SL, Sadeghian K, Kelley AE (1999) Spatial learning and performance in the radial arm maze is impaired after N-methyl-d-aspartate (NMDA) receptor blockade in striatal subregions. Behav Neurosci 113:703–717
Thinus-Blanc C, Durup M, Poucet B (1992) The spatial parameters encoded by hamsters during exploration: a further study. Behav Processes 26:43–57
Usiello A, Sargolini F, Roullet P, Ammassari-Teule M, Passino E, Oliverio A, Mele A (1998) N-Methyl-d-aspartate receptors in the nucleus accumbens are involved in detection of spatial novelty in mice. Psychopharmacology 137:175–183
Wang LY, Salter MW, MacDonald JF (1991) Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science 253:1132–1135
Wolf ME, Sun X, Mangiavacchi S, Chao SZ (2004) Psychomotor stimulants and neuronal plasticity. Neuropharmacology 47:61–79
Zham DS (1989) The ventral striatopallidal parts of the basal ganglia in the rat: compartimentation of the ventral pallidal efferents. Neuroscience 30:33–50
Acknowledgements
This study was supported by Cofin grants to A.O. and A.M. and an F.I.R.B. grant to A.O. and A.M. The authors would like to thank Mr. Tullio Riosa and Mr. Genesio Ricci for their technical assistance and Dr. Agu Pert and Dr. Claudio Castellano for their useful suggestions on a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferretti, V., Florian, C., Costantini, V.J.A. et al. Co-activation of glutamate and dopamine receptors within the nucleus accumbens is required for spatial memory consolidation in mice. Psychopharmacology 179, 108–116 (2005). https://doi.org/10.1007/s00213-005-2144-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2144-3