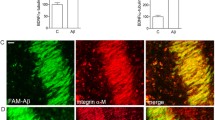
Abstract
Amyloid β-protein (Aβ), a putative pathogenic endotoxin involved in Alzheimer's disease, induces redistribution of glutamate transporters in astrocytes and promotes their pump activity. Because the transporters are assumed to protect neurons against excitotoxicity by removing extracellular glutamate, we hypothesized that Aβ alters the vulnerability of neurons to glutamate. Cerebrocortical neuron-astroglial co-cultures were exposed to glutamate, the concentration of which was selected so that only 20% of the neurons exhibited degeneration. When cultures were pre-treated with Aβ, exposure to the same "mild" glutamate concentration failed to damage neurons. The Aβ-induced protection was abolished by a glial glutamate transporter inhibitor. Thus, Aβ can alleviate excitotoxicity through glutamate transporter activity. The present results may challenge prevailing concepts that Aβ-induced neuron loss causes Alzheimer's dementia and also provide practical insights into neuro-glial interactions in glutamate toxicity.


Similar content being viewed by others
References
Abe K, Abe Y, Saito H (2000) Evaluation of l-glutamate clearance capacity of cultured rat cortical astrocytes. Biol Pharmaceut Bull 23:204–207
Brew H, Attwell D (1987) Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature 327:707–709
Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2:271–276
Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG (2000) A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease, Nature 408:975–979
Davies CA, Mann DM, Sumpter PQ, Yates PO (1987) A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci 78:151–164
Fitzjohn SM, Morton RA, Kuenzi F, Rosahl TW, Shearman M, Lewis H, Smith D, Reynolds DS, Davies CH, Collingridge GL, Seabrook GR (2001) Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J Neurosci 21:4691–469
Forloni G, Angeretti N, Chiesa R, Monzani E, Salmona M, Bugiani O, Tagliavini F (1993) Neurotoxicity of a prion protein fragment. Nature 362:543–546
Horn D, Levy N, Ruppin E (1996) Neuronal-based synaptic compensation: a computational study in Alzheimer's disease. Neural Comput 8:1227–1243
Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll R, Mucke L (1999) Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA 96:3228–3233
Ikegaya Y, Matsuura S, Ueno S, Baba A, Yamada MK, Nishiyama N, Matsuki N (2002) β-Amyloid enhances glial glutamate uptake activity and attenuates synaptic efficacy. J Biol Chem 277:32180–32186
Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R (2003) APP processing and synaptic function. Neuron 37:925–937
Kanai Y, Smith CP, Hediger MA (1993) A new family of neurotransmitter transporters: the high-affinity glutamate transporters. FASEB J 7:1450–1459
Larson J, Lynch G, Games D, Seubert P (1999) Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res 840:23–35
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1996) Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95:6448–6453
Matsuura S, Ikegaya Y, Yamada MK, Nishiyama N, Matsuki N (2002) Endothelin downregulates the glutamate transporter GLAST in cAMP-differentiated astrocytes in vitro. Glia 37:178–182
Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE (1992) β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci 12:376–389
Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F (1999) Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem 274:6483–6492
Nitta A, Itoh A, Hasegawa T, Nabeshima T (1994) β-Amyloid protein-induced Alzheimer's disease animal model. Neurosci Lett 170:63–66
Oleny JW (1989) Excitatory amino acids and neuropsychiatric disorders. Biol Psychiatry 26:505–525
Ruppin E, Reggia JA (1995) A neural model of memory impairment in diffuse cerebral atrophy. Br J Psychiatry 166:19–28
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P (1999) Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400:173–177
Selkoe DJ (2002) Alzheimer's disease is a synaptic failure. Science 298:789–791
Small DH, McLean CA (1999) Alzheimer's disease and the amyloid β protein: what is the role of amyloid? J Neurochem 73:443–449
Small DH, Mok SS, Bornstein JC (2001) Alzheimer's disease and Aβ toxicity: from top to bottom. Nat Rev Neurosci 2:595–598
Stéphan A, Laroche S, Davis S (2001) Generation of aggregated β-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci 21:5703–5714
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580
Yankner BA, Duffy LK, Kirschner DA (1990) Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science 250:279–282
Acknowledgements
The authors are grateful to Dr. T. Shirasawa (Tokyo Metropolitan Institute of Gerontology) for providing synthesized Aβ. This work was supported in part by Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Research Grant for Longevity Science (13-2) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baba, A., Mitsumori, K., Yamada, M.K. et al. β-Amyloid prevents excitotoxicity via recruitment of glial glutamate transporters. Naunyn-Schmiedeberg's Arch Pharmacol 368, 234–238 (2003). https://doi.org/10.1007/s00210-003-0792-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-003-0792-6