Abstract
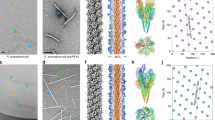
Ultrastructural studies using scanning electron microscopy (SEM), negative-staining transmission electron microscopy (TEM), and thin-sectioning TEM on four species of Spiroplasma, in vitro and/or in vivo, indicated that their helices commonly possess one tapered end (tip structure) and one blunt or round end. These tip structures appeared morphologically different from the rest of the helix, exhibiting an electron-dense conical or rod-shaped core. In thin sections of the midgut of the leafhopper Dalbulus elimatus, the tip structures of Spiroplasma kunkelii in the midgut lumen were mostly aligned between microvilli, perpendicular to the apical plasma membrane of epithelial cells. These tip structures appeared frequently attached or closely apposed to the plasma membrane, in which cup-shaped invaginations close to the tips were observed. Pleomorphic forms of spiroplasma, enclosed in membranous vesicles, were found in the cytoplasm of the midgut epithelial cells. These findings suggest that the tip structure may be involved in the orientation and attachment of spiroplasma helices in relation to their host cells, and thus may be functionally comparable to the “attachment organelle” of mycoplasmas. Additionally, pili-like structures were observed by negative-staining TEM on the surface of Spiroplasma melliferum, and in thin sections of S. kunkelii infecting the leafhopper vector Dalbulus gelbus.





Similar content being viewed by others
Abbreviations
- CSS :
-
Corn stunt spiroplasma
- SEM :
-
Scanning electron microscopy
- TBS :
-
Tris-buffered saline
- TEM :
-
Transmission electron microscopy
References
Bai X, Hogenhout SA (2002) A genome sequence survey of the mollicute corn stunt spiroplasma Spiroplasma kunkelii. FEMS Microbiol. Lett 210:7-17
Bové JM (1997) Spiroplasmas: Infectious agents of plants, arthropods and vertebrates. Wien Klin Wochenschr 109:604–612
Brenner C, Neyrolles O, Blanchard A (1996) Mycoplasmas and HIV infection: from epidemiology to their interaction with immune cells. Front Biosci 1:42–54
Carle P, Laigret F, Tully JG, Bové JM (1995) Heterogeneity of genome sizes with the genus Spiroplasma. Int J Syst Bacteriol 45:178–181
Clark TB (1984) Diversity of spiroplasma host-parasite relationships. Isr J Med Sci 20:995–997
Ebbert MA, Nault LR (1994) Survival in Dalbulus leafhopper vectors improves after exposure to maize stunting pathogens. Entomol Exp Appl 100:311–324
Fletcher J, Wayadande A, Melcher U (1998) The phytopathogenic mollicute-insect vector interface: A closer look. Phytopathology 88:1351–1358
Garnier M, Clerc M, Bové JM (1981) Growth and division of spiroplasmas: Morphology of Spiroplasma citri during growth in liquid medium. J Bacteriol 147:642–652
Gasparich GE (2002) Spiroplasmas: evolution, adaptation and diversity. Front Biosci 7:619–640
Humphery-Smith I, Chastel C, Grulet O, Le Goff F (1990) The infective cycle of Spiroplasma sabaudience in vitro in Aedes albopictus (C6/36) cells and its potential for the biological control of dengue virus. Zentralbl Bakteriol Mikrobiol Hyg Abt Suppl 20:922–924
Jordan RL, Konai M, Lee IM, Davis RE (1989) Species-specific and cross-reactive monoclonal antibodies to the plant-pathogenic spiroplasma Spiroplasma citri and Spiroplasma kunkelii. Phytopathology 79:880–887
Kwon MO, Wayadande AC, Fletcher J (1999) Spiroplasma citri movement into the intestines and salivary glands of its leafhopper vector, Circulifer tenellus. Phytopathology 89:1144–1151
Lee IM, Davis RE (1989) Serum-free media for cultivation of spiroplasmas. Can J Microbiol 35:1092–1099
Maniloff J (1988) Mycoplasma viruses. Crit Rev Microbiol 15:339–389
Marcone C, Neimark H, Ragozzino A, Lauer U, Seemüller E (1999) Chromosome sizes of phytoplasmas composing phylogenetic groups and subgroups. Phytopathology 89:805–810
Markham PG (1983) Spiroplasmas in leafhoppers: a review. Yale J Biol Med 56:745–751
Nault LR (1990) Evolution of an insect pest: Maize and the corn leafhopper, a case study. Maydica 35:165–175
Oshima K, Miyata S, Sawayanagi T, Kakizawa S, Nishigawa H, Jung HY, Furuki K, Yanazaki M, Suzuki S, Wei W, Kuboyama T, Ugaki M, Namba S (2002) Minimal set of metabolic pathways suggested from the genome of onion yellows phytoplasma. J Gen Pathol 68:225–236
Özbek E, Miller SA, Meulia T, Hogenhout SA (2003) Infection and replication sites of Spiroplasma kunkelii (Class: Mollicutes) in midgut and Malpighian tubules of the leafhopper Dalbulus maidis. J Invertebr Pathol 82:167–175
Phillips RN, Humphery-Smith I (1995) The histopathology of experimentally induced infections of Spiroplasma taiwanense (Class: Mollicutes) in Anopheles stephensi mosquitoes. J Invertebr Pathol 66:185–195
Razin S, Yogev D, Naot Y (1998) Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Rev 62:1094–1156
Rottem S (2003) Interaction of mycoplasmas with host cells. Physiol Rev 83:417–432
Sasaki Y, Ishikawa J, Yamashita A, Oshima K, Kenri T, Furuya K, Yoshino C, Horino A, Shiba T, Sasaki T, Hattori M (2002) The complete genomic sequence of Mycoplasma penetrans, an intracellular bacterial pathogen in humans. Nucleic Acids Res 30:5293–5300
Sha YH, Melcher U, Davis RE, Fletcher J (1995) Resistance of Spiroplasma citri lines to the virus SVTS2 is associated with integration of viral DNA sequences into host chromosomal and extrachromosomal DNA. Appl Environ Microbiol 61: 3950–3959
Snedecor GW, Cochran WG (1989) Statistical methods, 8th edn. Iowa State University Press, Ames, Iowa
Townsend R (1983) Viruses of Spiroplasma citri and their possible effects on pathogenicity. Yale J Biol Med 56:771–776
Trachtenberg S (1998) Mollicutes—wall-less bacteria with internal cytoskeletons. J Struct Biol 124:244–256
Trachtenberg S, Gilad R (2001) A bacterial linear motor: cellular and molecular organization of the contractile cytoskeleton of the helical bacterium Spiroplasma melliferum BC3. Mol Microbiol 41:827–848
Trachtenberg S, Andrews SB, Leapman RD (2003a) Mass distribution and spatial organization of the linear bacterial motor of Spiroplasma citri R8A2. J Bacteriol 185:1987–1994
Trachtenberg S, Gilad R, Geffen N (2003b) The bacterial linear motor of Spiroplasma melliferum BC3: from single molecules to swimming cells. Mol Microbiol 47:671–697
Tsai J (1979) Vector transmission of mycoplasmal agents of plant disease. In: Whitcomb RF, Tully JG (eds) The mycoplasmas III. Plant and insect mycoplasmas. Academic, New York, pp 266–307
Wayadande AC, Fletcher J (1995) Transmission of Spiroplasma citri lines and their ability to cross gut and salivary gland barriers within the leafhopper vector Circulifer tenellus. Phytopathology 85:1256–1259
Acknowledgements
This research was funded by the OSU-OARDC research enhancement and competitive grants program and the OARDC Molecular and Cellular Imaging Center (MCIC). We thank Dr. Charles R. Krause and Leona Horst (USDA, ARS, Wooster, OH) for their kind permission and help (respectively) in using the Hitachi 4700 SEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ammar, ED., Fulton, D., Bai, X. et al. An attachment tip and pili-like structures in insect- and plant-pathogenic spiroplasmas of the class Mollicutes. Arch Microbiol 181, 97–105 (2004). https://doi.org/10.1007/s00203-003-0630-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-003-0630-8