Abstract
Summary
Spinal cord injury (SCI) causes rapid and marked bone loss. The present study demonstrates that low-intensity vibration (LIV) improves selected biomarkers of bone turnover and gene expression and reduces osteoclastogenesis, suggesting that LIV may be expected to benefit to bone mass, resorption, and formation after SCI.
Introduction
Sublesional bone is rapidly and extensively lost following spinal cord injury (SCI). Low-intensity vibration (LIV) has been suggested to reduce loss of bone in children with disabilities and osteoporotic women, but its efficacy in SCI-related bone loss has not been tested. The purpose of this study was to characterize effects of LIV on bone and bone cells in an animal model of SCI.
Methods
The effects of LIV initiated 28 days after SCI and provided for 15 min twice daily 5 days each week for 35 days were examined in female rats with moderate severity contusion injury of the mid-thoracic spinal cord.
Results
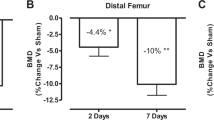
Bone mineral density (BMD) of the distal femur and proximal tibia declined by 5 % and was not altered by LIV. Serum osteocalcin was reduced after SCI by 20 % and was increased by LIV to a level similar to that of control animals. The osteoclastogenic potential of bone marrow precursors was increased after SCI by twofold and associated with 30 % elevation in serum CTX. LIV reduced the osteoclastogenic potential of marrow precursors by 70 % but did not alter serum CTX. LIV completely reversed the twofold elevation in messenger RNA (mRNA) levels for SOST and the 40 % reduction in Runx2 mRNA in bone marrow stromal cells resulting from SCI.
Conclusion
The findings demonstrate an ability of LIV to improve selected biomarkers of bone turnover and gene expression and to reduce osteoclastogenesis. The study indicates a possibility that LIV initiated earlier after SCI and/or continued for a longer duration would increase bone mass.






Similar content being viewed by others
References
Dudley-Javoroski S, Shields RK (2008) Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev 45:283–296
Qin W, Bauman WA, Cardozo C (2010) Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci 1211:66–84
Biering-Sorensen B, Kristensen IB, Kjaer M, Biering-Sorensen F (2009) Muscle after spinal cord injury. Muscle Nerve 40:499–519
Qin W, Bauman WA, Cardozo CP (2010) Evolving concepts in neurogenic osteoporosis. Curr Osteoporos Rep 8:212–218
Bauman WA, Cardozo C (2013) Spinal cord injury: pathophysiology and clinical issues. In: Rosen C (ed) Primer on the metabolic bone diseases and disorders of bone metabolism, 8th edn. American Society for Bone and Mineral Research, Washington D.C., pp 1018–1027
Giangregorio L, McCartney N (2006) Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 29:489–500
Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF (2000) Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 27:305–309
Logan WC Jr, Sloane R, Lyles KW, Goldstein B, Hoenig HM (2008) Incidence of fractures in a cohort of veterans with chronic multiple sclerosis or traumatic spinal cord injury. Arch Phys Med Rehabil 89:237–243
Garland DE, Adkins RH, Kushwaha V, Stewart C (2004) Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med 27:202–206
Akhigbe T, Chin AS, Svircev JN, Hoenig H, Burns SP, Weaver FM, Bailey L, Carbone L (2013) A retrospective review of lower extremity fracture care in patients with spinal cord injury. J Spinal Cord Med
Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, Lazzari AA, Garshick E (2009) Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int 20:385–392
Jiang SD, Jiang LS, Dai LY (2007) Changes in bone mass, bone structure, bone biomechanical properties, and bone metabolism after spinal cord injury: a 6-month longitudinal study in growing rats. Calcif Tissue Int 80:167–175
Morse L, Teng YD, Pham L, Newton K, Yu D, Liao WL, Kohler T, Muller R, Graves D, Stashenko P, Battaglino R (2008) Spinal cord injury causes rapid osteoclastic resorption and growth plate abnormalities in growing rats (SCI-induced bone loss in growing rats). Osteoporos Int 19:645–652
Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K (2001) Anabolism. Low mechanical signals strengthen long bones. Nature 412:603–604
Xie L, Rubin C, Judex S (2008) Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol 104:1056–1062
Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z (2004) Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res 19:360–369
Reyes ML, Hernandez M, Holmgren LJ, Sanhueza E, Escobar RG (2011) High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. J Bone Miner Res 26:1759–1766
Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K (2004) Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 19:343–351
Beck BR, Norling TL (2010) The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am J Phys Med Rehabil 89:997–1009
Von Stengel S, Kemmler W, Bebenek M, Engelke K, Kalender WA (2011) Effects of whole-body vibration training on different devices on bone mineral density. Med Sci Sports Exerc 43:1071–1079
von Stengel S, Kemmler W, Engelke K, Kalender WA (2011) Effects of whole body vibration on bone mineral density and falls: results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos Int 22:317–325
Garman R, Gaudette G, Donahue LR, Rubin C, Judex S (2007) Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res 25:732–740
Judex S, Lei X, Han D, Rubin C (2007) Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech 40:1333–1339
Ozcivici E, Luu YK, Rubin CT, Judex S (2010) Low-level vibrations retain bone marrow’s osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS ONE 5:e11178
Ness LL, Field-Fote EC (2009) Whole-body vibration improves walking function in individuals with spinal cord injury: a pilot study. Gait Posture 30:436–440
Herrero AJ, Menendez H, Gil L, Martin J, Martin T, Garcia-Lopez D, Gil-Agudo A, Marin PJ (2011) Effects of whole-body vibration on blood flow and neuromuscular activity in spinal cord injury. Spinal Cord 49:554–559
Ness LL, Field-Fote EC (2009) Effect of whole-body vibration on quadriceps spasticity in individuals with spastic hypertonia due to spinal cord injury. Restor Neurol Neurosci 27:621–631
Murillo N, Kumru H, Vidal-Samso J, Benito J, Medina J, Navarro X, Valls-Sole J (2011) Decrease of spasticity with muscle vibration in patients with spinal cord injury. Clin Neurophysiol 122:1183–1189
Bauman WA, Schnitzer TJ, Chen D (2010) Management of osteoporosis after spinal cord injury: what can be done? Point/counterpoint. PM R 2:566–572
Asselin P, Spungen AM, Muir JW, Rubin CT, Bauman WA (2011) Transmission of low-intensity vibration through the axial skeleton of persons with spinal cord injury as a potential intervention for preservation of bone quantity and quality. J Spinal Cord Med 34:52–59
Davis R, Sanborn C, Nichols D, Bazett-Jones DM, Dugan EL (2010) The effects of whole body vibration on bone mineral density for a person with a spinal cord injury: a case study. Adapt Phys Act Q: APAQ 27:60–72
Pinzon A, Marcillo A, Quintana A, Stamler S, Bunge MB, Bramlett HM, Dietrich WD (2008) A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model. Brain Res 1243:146–151
Voor MJ, Brown EH, Xu Q, Waddell SW, Burden RL, Burke DA, Magnuson DS (2012) Bone loss following spinal cord injury in a rat model. J Neurotrauma 29:1676
Wirth F, Schempf G, Stein G, Wellmann K, Manthou M, Scholl C, Sidorenko M, Semler O, Eisel L, Harrach R, Angelova S, Jaminet P, Ankerne J, Ashrafi M, Ozsoy O, Ozsoy U, Schubert H, Abdulla D, Dunlop SA, Angelov DN, Irintchev A, Schonau E (2013) Whole-body vibration improves functional recovery in spinal cord injured rats. J Neurotrauma 30:453–468
Sun L, Pan J, Peng Y, Wu Y, Li J, Liu X, Qin Y, Bauman WA, Cardozo C, Zaidi M, Qin W (2013) Anabolic steroids reduce spinal cord injury-related bone loss in rats associated with increased Wnt signaling. J Spinal Cord Med 36:616–622
Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S (2006) Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone 39:1059–1066
Wysocki A, Bulter M, Shamliyan T, Kane R (2011) Whole-body vibration therapy for osteoporosis. AHRQ Publication No. 11(12)-EHC083-EF
Sen B, Xie Z, Case N, Styner M, Rubin CT, Rubin J (2011) Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech 44:593–599
Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139:244–256
Qin W, Sun L, Cao J, Peng Y, Collier L, Wu Y, Creasey G, Li J, Qin Y, Jarvis J, Bauman WA, Zaidi M, Cardozo C (2013) The central nervous system (CNS)-independent anti-bone-resorptive activity of muscle contraction and the underlying molecular and cellular signatures. J Biol Chem 288:13511–13521
Cardozo CP, Qin W, Peng Y, Liu X, Wu Y, Pan J, Bauman WA, Zaidi M, Sun L (2010) Nandrolone slows hindlimb bone loss in a rat model of bone loss due to denervation. Ann N Y Acad Sci 1192:303–306
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Wu Y, Zhao J, Zhao W, Pan J, Bauman WA, Cardozo CP (2012) Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J Neurotrauma 29:1663–1675
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Bauman WA, Cardozo C (2013) Immobilization osteoporosis. In: Marcus R, Nelson D, Rosen CJ (eds) Osteoporosis, 4th edn. Academic
Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, Gerard RD, Rothermel BA, Hill JA (2006) Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation 114:1159–1168
Bassel-Duby R, Olson EN (2006) Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75:19–37
Schiaffino S (2010) Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf) 199:451–463
Schoenau E (2005) From mechanostat theory to development of the “functional muscle-bone-unit”. J Musculoskelet Neuronal Interact 5:232–238
Rittweger J, Beller G, Ehrig J, Jung C, Koch U, Ramolla J, Schmidt F, Newitt D, Majumdar S, Schiessl H, Felsenberg D (2000) Bone-muscle strength indices for the human lower leg. Bone 27:319–326
Arija-Blazquez A, Ceruelo-Abajo S, Diaz-Merino MS, Godino-Duran JA, Martinez-Dhier L, Florensa-Vila J (2013) Time-course response in serum markers of bone turnover to a single-bout of electrical stimulation in patients with recent spinal cord injury. Eur J Appl Physiol 113:89–97
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33:747–783
Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68:577–589
Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39:91–97
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ (2011) Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE 6:e25900
Kearns AE, Khosla S, Kostenuik PJ (2008) Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 29:155–192
McKeehen JN, Novotny SA, Baltgalvis KA, Call JA, Nuckley DJ, Lowe DA (2013) Adaptations of mouse skeletal muscle to low-intensity vibration training. Med Sci Sports Exerc 45:1051–1059
Acknowledgments
This work was supported by the Veterans Health Administration, Rehabilitation Research and Development Service (B9212-C and B0687-R), Biomedical Laboratory Research and Development Service (BX000521), the Department of Defense (#SC090504), and The Miami Project to Cure Paralysis. We wish to thank Drs. Edelle Field-Fote and Mark Nash for critical reading of the manuscript.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 18 kb)
Supplemental Figure 1
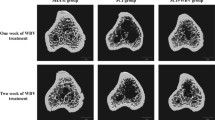
Histomorphometric analysis of bone at 63 days after a moderate mid-throacic contusion SCI. A. Representative sections of trabecular bone from the femoral metaphysis immunostained for TRAP. Note the reddish areas of TRAP+ staining on trabecular surfaces representing osteoclasts. B. Histomorphometric quantification of osteoclast numbers and surface: osteoclast surface per bone surface (Oc.S/Bs %); surface eroded by osteoclasts per bone surface (ES /Bs%), and number of osteoclasts per tissue area of interest (N. Oc/ T. Ar, Oc/mm2). Data are expressed as mean ± SEM. N = 5–6 per group. Significance of differences was determined using one-way ANOVA with a Newman–Keuls test post hoc. **P < 0.01 versus the indicated group; NS. No statistic significance. (GIF 277 kb)
Rights and permissions
About this article
Cite this article
Bramlett, H.M., Dietrich, W.D., Marcillo, A. et al. Effects of low intensity vibration on bone and muscle in rats with spinal cord injury. Osteoporos Int 25, 2209–2219 (2014). https://doi.org/10.1007/s00198-014-2748-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2748-8