Abstract
Summary
Changes in bone mineral density and bone strength following treatment with zoledronic acid (ZOL) were measured by quantitative computed analysis (QCT) or dual-energy X-ray absorptiometry (DXA). ZOL treatment increased spine and hip BMD vs placebo, assessed by QCT and DXA. Changes in trabecular bone resulted in increased bone strength.
Introduction
To investigate bone mineral density (BMD) changes in trabecular and cortical bone, estimated by quantitative computed analysis (QCT) or dual-energy X-ray absorptiometry (DXA), and whether zoledronic acid 5 mg (ZOL) affects bone strength.
Methods
In 233 women from a randomized, controlled trial of once-yearly ZOL, lumbar spine, total hip, femoral neck, and trochanter were assessed by DXA and QCT (baseline, Month 36). Mean percentage changes from baseline and between-treatment differences (ZOL vs placebo, t-test) were evaluated.
Results
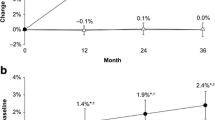
Mean between-treatment differences for lumbar spine BMD were significant by DXA (7.0%, p < 0.01) and QCT (5.7%, p < 0.0001). Between-treatment differences were significant for trabecular spine (p = 0.0017) [non-parametric test], trabecular trochanter (10.7%, p < 0.0001), total hip (10.8%, p < 0.0001), and compressive strength indices at femoral neck (8.6%, p = 0.0001), and trochanter (14.1%, p < 0.0001).
Conclusions
Once-yearly ZOL increased hip and spine BMD vs placebo, assessed by QCT vs DXA. Changes in trabecular bone resulted in increased indices of compressive strength.

Similar content being viewed by others
References
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Cheng X, Li J, Lu Y, Keyak J, Lang T (2007) Proximal femoral density and geometry measurements by quantitative computed tomography: association with hip fracture. Bone 40:169–174
Lang T, Leblanc A, Evans H, Lu Y, Genant H, Yu A (2004) Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 19:1006–1012
Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ (2005) One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353:555–565
McClung MR, San MJ, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768
Genant HK, Lang T, Fuerst T, Pinette KV, Zhou C, Thiebaud D, Diez-Perez A (2004) Treatment with raloxifene for 2 years increases vertebral bone mineral density as measured by volumetric quantitative computed tomography. Bone 35:1164–1168
Lotz JC, Hayes WC (1990) The use of quantitative computed tomography to estimate risk of fracture of the hip from falls. J Bone Joint Surg Am 72:689–700
Buckley JM, Loo K, Motherway J (2007) Comparison of quantitative computed tomography-based measures in predicting vertebral compressive strength. Bone 40:767–774
Acknowledgements
Dr. Boonen is senior clinical investigator of the Fund for Scientific Research, Flanders, Belgium (F.W.O.-Vlaanderen). Dr. Isra Saeed at UCSF carried out the QCT image analysis and provided substantial support in managing data for the Reading centre. Special thanks are due Zeb Horowitz (now at Savient Pharmaceuticals), John Orloff (Novartis), and the following: Steering Committee members—Dennis Black, Steven Cummings, Pierre Delmas, Richard Eastell, Ian Reid, Steven Boonen, Jane Cauley, Felicia Cosman, Péter Lakatos, Ping C. Leung, Zulema Man, Erik Fink Eriksen (Novartis), Peter Mesenbrink (Novartis); Past Steering Committee members—Edith Lau, Saloman Jasqui, Carlos Mautalen, Theresa Rosario-Jansen (Novartis), John Caminis (Novartis); Data Safety Monitoring Board—Lawrence Raisz (chairman), Peter Bauer, Juliet Compston, David DeMets, Raimund Hirschberg, Olof Johnell, Stuart Ralston, Robert Wallace; DSMB Consultants—Michael Farkough; Novartis—Mary Flood; University of California, San Francisco (UCSF) Coordinating Center—Douglas Bauer, Lisa Palermo; UCSF Radiology (QCT analysis)—Thomas Lang. We are indebted to the HORIZON-PFT Clinical Site Investigators: Argentina: Eduardo Kerzberg, Zulema Man, Carlos Mautalen, Maria Ridruejo, Guillermo Tate, Jorge Velasco; Australia: Michael Hooper, Mark Kotowicz, Peter Nash, Richard Prince, Anthony Roberts, Philip Sambrook; Austria: Harald Dobnig, Gerd Finkenstedt, Guenter Hoefle, Klaus Klaushofer, Martin Pecherstorfer, Peter Peichl; Belgium: Jean Body, Steven Boonen, Jean-Pierre Devogelaer, Piet Geusens, Jean Kaufman; Brazil: João Brenol, Jussara Kochen, Rubem Lederman, Sebastiao Radominski, Vera Szejnfeld, Cristiano Zerbini; Canada: Jonathan Adachi, Jacques Brown, Denis Choquette, David Hanley, Robert Josse, David Kendler, Richard Kremer, Frederic Morin, Wojciech Olszynski, Alexandra Papaioannou, Chiu KinYuen; China: Baoying Chen, Shouqing Lin; Colombia: Nohemi Casas, Monique Chalem, Juan Jaller, Jose Molina; Finland: Hannu Aro, Jorma Heikkinen, Heikki Kröger, Lasse Mäkinen, Juha Saltevo, Jorma Salmi, Matti Välimäki; France: Claude-Laurent Benhamou, Pierre Delmas, Patrice Fardellone, Georges Werhya; Germany: Bruno Allolio, Dieter Felsenberg, Joachim Happ, Manfred Hartard, Johannes Hensen, Peter Kaps, Joern Kekow, Ruediger Moericke, Bernd Ortloff, Peter Schneider, Siegfried Wassenberg; Hong Kong: Ping Chung Leung; Hungary: Adam Balogh, Bela Gomor, Tibor Hidvégi, Laszlo Koranyi, Péter Lakatos, Gyula Poór, Zsolt Tulassay; Israel: Rivka Dresner Pollak, Varda Eshed, A. Joseph Foldes, Sophia Ish-Shalom, Iris Vered, Mordechai Weiss; Italy: Silvano Adami, Antonella Barone, Gerolamo Bianchi, Sandro Giannini, Giovanni Carlo Isaia, Giovanni Luisetto, Salvatore Minisola, Nicola Molea, Ranuccio Nuti, Sergio Ortolani, Mario Passeri, Alessandro Rubinacci, Bruno Seriolo, Luigi Sinigaglia; Korea (Republic of): Woong-Hwan Choi, Moo-II Kang, Ghi-Su Kim, Hye-Soon Kim, Yong-Ki Kim, Sung-Kil Lim, Ho-Young Son, Hyun-Koo Yoon; Mexico: Carlos Abud, Pedro Garcia, Salomon Jasqui, Luis Ochoa, Javier Orozco, Javier Santos; New Zealand: Ian Reid; Norway: Sigbjørn Elle, Johan Halse, Arne Høiseth, Hans Olav, Høivik Ingun Røed, Arne Skag, Jacob Stakkestad, Unni Syversen; Poland: Janusz Badurski, Edward Czerwinski, Roman Lorenc, Ewa Marcinowska-Suchowierska, Andrzej Sawicki, Jerzy Supronik; Russia: Eduard Ailamazyan, Lidiya Benevolenskaya, Alexander Dreval, Leonid Dvoretsky, Raisa Dyomina, Vadim Mazurov, Galina Melnichenko, Ashot Mkrtoumyan, Alexander Orlov-Morozov, Olga Ostroumova, Eduard Pikhlak, Tatiana Shemerovskaya, Nadezhda Shostak, Irina Skripnikova, Vera Smetnik, Evgenia Tsyrlina, Galina Usova, Alsu Zalevskaya, Irina Zazerskaya, Eugeny Zotkin; Sweden: Osten Ljunggren, Johan Lofgren, Mats Palmér, Maria Saaf, Martin Stenström; Switzerland: Paul Hasler, Olivier Lamy, Kurt Lippuner, Claude Merlin, René Rizzoli, Robert Theiler, Alan Tyndall, Daniel Uebelhart; Taiwan: Jung-Fu Chen, Po-Quang Chen, Lin-show Chin, Jawl-Shan Hwang, Tzay-Shing Yang, Mayuree Jirapinyo; Thailand: Mayuree Jirapinyo, Rojanasthien Sattaya, Sutin Sriussadaporn, Soontrapa Supasin, Nimit Taechakraichana, Kittisak Wilawan; United Kingdom: Hugh Donnachie, Richard Eastell, William Fraser, Alistair McLellan, David Reid; United States: John Abruzzo, Ronald Ackerman, Robert Adler, John Aloia, Charles Birbara, Barbara Bode, Henry Bone, Donald Brandon, Jane Cauley, Felicia Cosman, Daniel Dionne, Robert Downs, Jr., James Dreyfus, Victor Elinoff. Ronald Emkey, Joseph Fanciullo, Darrell Fiske, Palmieri Genaro, M. Gollapudi, Richard Gordon, James Hennessey, Paul Howard, Karen Johnson, Conrad Johnston, Risa Kagan, Shelly Kafka, Jeffrey Kaine, Terry Klein, William Koltun, Meryl Leboff, Bruce Levine, E. Michael Lewiecki, Cora Elizabeth Lewis, Angelo Licata, Michael Lillestol, Barry Lubin, Raymond Malamet, Antoinette Mangione, Velimir Matkovic, Daksha Mehta, Paul Miller, Sam Miller, Frederik T. Murphy, Susan Nattrass, David Podlecki, Christopher Recknor, Clifford Rosen, Daniel Rowe, Robert Rude, Thomas Schnitzer, Yvonne Sherrer, Stuart Silverman, Kenna Stephenson, Barbara Troupin, Joseph Tucci, Reina Villareal, Nelson Watts, Richard Weinstein, Robert Weinstein, Michael Weitz, Richard White.
Conflicts of interest
Dr. Eastell serves as a consultant, has received honoraria for speaking, and has received grant funding from Novartis, Amgen, Sanofi-Aventis, Lilly, Organon, Pfizer, and Procter & Gamble Pharmaceuticals. Dr. Lang serves as a consultant for Merck and has received grant funding from Novartis. Dr. Boonen serves as a consultant, has received honoraria for speaking, and has received grant funding from Novartis. Dr. Cummings reports no conflict of interest. Dr. Cauley has received research grants from Merck, Eli Lilly, Pfizer, Novartis and Procter & Gamble, and has received honoraria from Novartis. Dr. Horowitz was an employee of Novartis until 2003. He has no other conflict of interest. Dr. Kerzberg has no conflict of interest. Dr. Bianchi has received consulting fees from Novartis. Dr. Kendler serves on advisory boards or as a consultant for Novartis, Servier, Eli Lilly, Amgen, Wyeth and Nycomed. Dr. Leung has no conflict of interest. Dr. Man has received honoraria for speaking from Sanofi-Aventis, Roche, Merck and Novartis. Dr. Mesenbrink is an employee of Novartis Pharmaceuticals Corporation who funded the study and owns stock in the company. Dr. Eriksen serves as a consultant and has received honoraria for speaking from Novartis. Dr. Black serves as a consultant for Nycomed and Zosano, and has research contracts with Novartis and Roche.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
P. D. Delmas deceased
Supported by Novartis Pharma
Rights and permissions
About this article
Cite this article
Eastell, R., Lang, T., Boonen, S. et al. Effect of once-yearly zoledronic acid on the spine and hip as measured by quantitative computed tomography: results of the HORIZON Pivotal Fracture Trial. Osteoporos Int 21, 1277–1285 (2010). https://doi.org/10.1007/s00198-009-1077-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-1077-9