Abstract
Introduction and hypothesis
A method was developed using 3D stress magnetic resonance imaging (MRI) and was piloted to test hypotheses concerning changes in apical ligament lengths and lines of action from rest to maximal Valsalva.
Methods
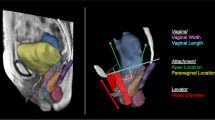
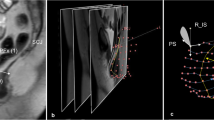
Ten women with (cases) and ten without (controls) pelvic organ prolapse (POP) were selected from an ongoing case–control study. Supine, multiplanar stress MRI was performed at rest and at maximal Valsalva and was imported into 3D Slicer v. 3.4.1 and aligned. The 3D reconstructions of the uterus and vagina, cardinal ligament (CL), deep uterosacral ligament (USLd), and pelvic bones were created. Ligament length and orientation were then measured.
Results
Adequate ligament representations were possible in all 20 study participants. When cases were compared with controls, the curve length of the CL at rest was 71 ±16 mm vs. 59 ± 9 mm (p = 0.051), and the USLd was 38 ± 16 mm vs. 36 ± 11 mm (p = 0.797). Similarly, the increase in CL length from rest to strain was 30 ± 16 mm vs. 15 ± 9 mm (p = 0.033), and USLd was 15 ± 12 mm vs. 7 ± 4 mm (p = 0.094). Likewise, the change in USLd angle was significantly different from CL (p < 0.001).
Conclusions
This technique allows quantification of 3D geometry at rest and at strain. In our pilot sample, at maximal Valsalva, CL elongation was greater in cases than controls, whereas USLd was not; CL also exhibited greater changes in ligament length, and USLd exhibited greater changes in ligament inclination angle.






Similar content being viewed by others
References
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89(4):501–506
Boyles SH, Weber AM, Meyn L (2003) Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol 188(1):108–115
Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS (2001) Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol 98(4):646–651
Summers A, Winkel LA, Hussain HK, DeLancey JO (2006) The relationship between anterior and apical compartment support. Am J Obstet Gynecol 194(5):1438–1443
Chen L, Ramanah R, Hsu Y, Ashton-Miller JA, Delancey JO (2013) Cardinal and deep uterosacral ligament lines of action: MRI based 3D technique development and preliminary findings in normal women. Int Urogynecol J 24(1):37–45
Larson KA, Hsu Y, Chen L, Ashton-Miller JA, DeLancey JO (2010) Magnetic resonance imaging-based three-dimensional model of anterior vaginal wall position at rest and maximal strain in women with and without prolapse. Int Urogynecol J 21(9):1103–1109
Larson KA, Luo J, Guire KE, Chen L, Ashton-Miller JA, DeLancey JO (2012) 3D analysis of cystoceles using magnetic resonance imaging assessing midline, paravaginal, and apical defects. Int Urogynecol J 23(3):285–293
Luo J, Larson KA, Fenner DE, Ashton-Miller JA, Delancey JO (2012) Posterior vaginal prolapse shape and position changes at maximal Valsalva seen in 3-D MRI-based models. Int Urogynecol J 23(9):1301–1306
Ramanah R, Berger MB, Chen LY, Riethmuller D, DeLancey JOL (2012) See it in 3D! Researchers examined structural links between the cardinal and uterosacral ligaments. Am J Obstet Gynecol 207(5):437.e1–437.e7
Campbell RM (1950) The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol 59(1):1–12
Range RL, Woodburne RT (1964) The gross and microscopic anatomy of the transverse cervical ligament. Am J Obstet Gynecol 90:460–467
Cole EE, Leu PB, Gomelsky A, Revelo P, Shappell H, Scarpero HM, Dmochowski RR (2006) Histopathological evaluation of the uterosacral ligament: is this a dependable structure for pelvic reconstruction? BJU Int 97(2):345–348
Ramanah R, Berger MB, Parratte BM, DeLancey JO (2012) Anatomy and histology of apical support: a literature review concerning cardinal and uterosacral ligaments. Int Urogynecol J 23(11):1483–1494
Tunn R, DeLancey JO, Quint EE (2001) Visibility of pelvic organ support system structures in magnetic resonance images without an endovaginal coil. Am J Obstet Gynecol 184(6):1156–1163
Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ (2004) Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol 190(1):20–26
Lee RA (1992) Atlas of gynecologic surgery. WB Saunders, Philadelphia
Shull BL, Bachofen C, Coates KW, Kuehl TJ (2000) A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol 183(6):1365–1374
Acknowledgments
We gratefully acknowledge support from the National Institutes of Health, Office for Research on Women’s Health, Specialized Center of Research: Sex and Gender Factors Affecting Women’s Health, Grant P50 HD 044406, and NIH R01 HD 038665.
Conflicts of interest
Dr. John O. DeLancey and Dr. James A. Ashton-Miller have no directly related conflicts of interest for this study. The University of Michigan received funding from Johnson & Johnson, American Medical Systems, Kimberly-Clark Corporation, Proctor & Gamble, and Boston Scientific Corporation as partial salary support for research unrelated to the topic of this paper. They received an honorarium and travel reimbursement for giving an invited research seminar at Johnson & Johnson.
Dr. Jiajia Luo has no directly related conflicts of interest for this study, but his doctoral studies were partially funded by American Medical Systems and Kimberly Clark Corporation unrelated to the topic of this paper, and he currently receives research support from Boston Scientific Corporation unrelated to the topic of this paper.
Dr. Luyun Chen received research support from American Medical Systems unrelated to the topic of this paper.
Dr. Cornelia Betschart received research support from the Swiss National Science Foundation unrelated to the topic of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, J., Betschart, C., Chen, L. et al. Using stress MRI to analyze the 3D changes in apical ligament geometry from rest to maximal Valsalva: a pilot study. Int Urogynecol J 25, 197–203 (2014). https://doi.org/10.1007/s00192-013-2211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-013-2211-y