Abstract
Aims/hypothesis
Excessive hepatic glucose production is a hallmark of insulin resistance in type 2 diabetes. The cAMP responsive transcription factor cAMP responsive element binding protein (CREB), thought to be a key activator of the hepatic gluconeogenic gene regulation programme, has been suggested as a therapeutic target to reduce glucose output by the liver. Here, we test directly the requirement for hepatocytic CREB for the maintenance of glucose homeostasis.
Methods
We derived mice with a Creb (also known as Creb1) loxP allele for conditional, cell-type specific gene ablation. Hepatocyte-specific deletion of Creb was induced by injecting Creb loxP/loxP mice with Cre recombinase expression adeno-associated virus.
Results
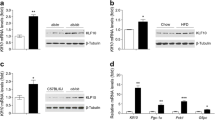
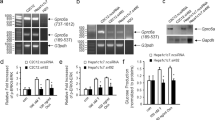
Strikingly, we found no difference in fed and fasted glucose levels, or in glucose, insulin and glucagon tolerance in mice fed a normal chow or a high-fat diet. In addition, mRNA levels of liver-specific genes, including several CREB target genes involved in gluconeogenesis, were not affected by CREB deficiency in the liver.
Conclusion/interpretation
Our data show that CREB has no non-redundant functions in hepatic glucose metabolism, and is therefore not likely to be a useful target for the development of glucose-lowering drugs.




Similar content being viewed by others
Abbreviations
- AAV:
-
Adeno-associated virus
- ACREB:
-
Acidic cAMP responsive element binding protein
- ASO:
-
Antisense oligonucleotide
- CRE:
-
cAMP responsive element
- CREB:
-
cAMP responsive element binding protein
- ES:
-
Embryonic stem
- GFP:
-
Green fluorescent protein
- HNF4α:
-
Hepatocyte nuclear factor 4α
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
References
Dwarki VJ, Montminy M, Verma IM (1990) Both the basic region and the 'leucine zipper' domain of the cyclic AMP response element binding (CREB) protein are essential for transcriptional activation. EMBO J 9:225–232
Zhang X, Odom DT, Koo S-H et al (2005) Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A 102:4459–4464
Everett LJ, Lay JL, Lukovac S et al (2013) Integrative genomic analysis of CREB defines a critical role for transcription factor networks in mediating the fed/fasted switch in liver. BMC Genomics 14:337
Herzig S, Long F, Jhala US et al (2001) CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183
Zhou XY, Shibusawa N, Naik K et al (2004) Insulin regulation of hepatic gluconeogenesis through phosphorylation of CREB-binding protein. Nat Med 10:633–637
Koo S-H, Flechner L, Qi L et al (2005) The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437:1109–1111
Le Lay J, Tuteja G, White P, Dhir R, Ahima R, Kaestner KH (2009) CRTC2 (TORC2) Contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose hhomeostasis. Cell Metab 10:55–62
Gastaldelli A, Baldi S, Pettiti M et al (2000) Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes 49:1367–1373
Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI (1992) Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Investig 90:1323–1327
DeFronzo RA, Ferrannini E, Simonson DC (1989) Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metab Clin Exp 38:387–395
Herzig S, Hedrick S, Morantte I, Koo S-H, Galimi F, Montminy M (2003) CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-[gamma]. Nature 426:190–193
Erion DM, Ignatova ID, Yonemitsu S et al (2009) Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab 10:499–506
Copeland NG, Jenkins NA, Court DL (2001) Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet 2:769–779
Holzenberger M, Lenzner C, Leneuve P et al (2000) Cre-mediated germline mosaicism: a method allowing rapid generation of several alleles of a target gene. Nucl Acids Res 28:e92–e92
Lu M, Wan M, Leavens KF et al (2012) Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med 18:388–395
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. PNAS 98:5116–5121
Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C (1998) A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol 18:967–977
Woolf TM, Melton DA, Jennings CG (1992) Specificity of antisense oligonucleotides in vivo. PNAS 89:7305–7309
Fisher AA, Ye D, Sergueev DS, Fisher MH, Shaw BR, Juliano RL (2002) Evaluating the specificity of antisense oligonucleotide conjugates a DNA array analysis. J Biol Chem 277:22980–22984
Hummler E, Cole TJ, Blendy JA et al (1994) Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci U S A 91:5647–5651
Acknowledgements
The authors would like to thank J. Richa (Transgenic Chimeric Mouse Facility of the University of Pennsylvania, Philadelphia, PA, USA) for blastocyst injection, A. Sandhu (Penn Vector Core, University of Pennsylvania, Philadelphia, PA, USA) for production of AAV, J. Schug and L. Everett (University of Pennsylvania, Philadelphia, PA, USA) for helpful comments on the design and analysis of mRNA expression profiling, and D. Martinez, N. Panackal and S. Agrio (Pathology Core of the Joseph Stokes Jr Research Institute, Children’s Hospital of Philadelphia, Philadelphia, PA, USA) for histological services.
Funding
This research was supported by the National Institutes of Health (NIH) grant no. P01-DK049210. The Transgenic Chimeric Mouse Facility of the University of Pennsylvania was supported by NIH grant no. P30-DK19525.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
JL performed experiment and data interpretation and derived the Creb conditional gene ablation model. DL performed experiments, acquired data, and performed data analysis and interpretation for characterising the mouse. KHK designed the study and participated in interpretation and discussion of the data. DL wrote the manuscript. JL and KHK carried out critical revisions of the manuscript. DL and KHK are responsible for the integrity of the work as a whole. All authors approved the final versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, D., Le Lay, J. & Kaestner, K.H. The transcription factor CREB has no non-redundant functions in hepatic glucose metabolism in mice. Diabetologia 57, 1242–1248 (2014). https://doi.org/10.1007/s00125-014-3203-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3203-2