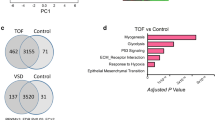
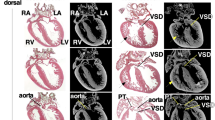
Abstract
Chamber-specific and temporally regulated perinatal cardiac growth and maturation is critical for functional adaptation of the heart and may be altered significantly in response to perinatal stress, such as systemic hypoxia (hypoxemia), leading to significant pathology, even mortality. Understanding transcriptome regulation of neonatal heart chambers in response to hypoxemia is necessary to develop chamber-specific therapies for infants with cyanotic congenital heart defects (CHDs). We sought to determine chamber-specific transcriptome programming during hypoxemic perinatal circulatory transition. We performed transcriptome-wide analysis on right ventricle (RV) and left ventricle (LV) of postnatal day 3 (P3) mouse hearts exposed to perinatal hypoxemia. Hypoxemia decreased baseline differences between RV and LV leading to significant attenuation of ventricular patterning (AVP), which involved several molecular pathways, including Wnt signaling suppression and cell cycle induction. Notably, robust changes in RV transcriptome in hypoxemic condition contributed significantly to the AVP. Remarkably, suppression of epithelial mesenchymal transition (EMT) and dysregulation of the TP53 signaling were prominent hallmarks of the AVP genes in neonatal mouse heart. Furthermore, members of the TP53-related gene family were dysregulated in the hypoxemic RVs of neonatal mouse and cyanotic Tetralogy of Fallot hearts. Integrated analysis of chamber-specific transcriptome revealed hypoxemia-specific changes that were more robust in RVs compared with LVs, leading to previously uncharacterized AVP induced by perinatal hypoxemia. Remarkably, reprogramming of EMT process and dysregulation of the TP53 network contributed to transcriptome remodeling of neonatal heart during hypoxemic circulatory transition. These insights may enhance our understanding of hypoxemia-induced pathogenesis in newborn infants with cyanotic CHD phenotypes.
Key messages
-
During perinatal circulatory transition, transcriptome programming is a major driving force of cardiac chamber-specific maturation and adaptation to hemodynamic load and external environment.
-
During hypoxemic perinatal transition, transcriptome reprogramming may affect chamber-specific growth and development, particularly in newborns with congenital heart defects (CHDs).
-
Chamber-specific transcriptome changes during hypoxemic perinatal transition are yet to be fully elucidated.
-
Systems-based analysis of hypoxemic neonatal hearts at postnatal day 3 reveals chamber-specific transcriptome signatures during hypoxemic perinatal transition, which involve attenuation of ventricular patterning (AVP) and repression of epithelial mesenchymal transition (EMT).
-
Key regulatory circuits involved in hypoxemia response were identified including suppression of Wnt signaling, induction of cellular proliferation and dysregulation of TP53 network.





Similar content being viewed by others
Abbreviations
- CHD:
-
Congenital heart defect
- RVOT:
-
Right ventricle outflow tract
- TOF:
-
Tetralogy of Fallot
- EMT:
-
Epithelial mesenchymal transition
- AVP:
-
Attenuation of ventricular patterning
- CMC:
-
Cardiomyocyte
References
Zhao Y, Kang X, Gao F, Guzman A, Lau RP, Biniwale R, Wadehra M, Reemtsen B, Garg M, Halnon N, Quintero-Rivera F, Arsdell GV, Giovanni C, Nelson SF, Touma, M & the UCLA Congenital Heart Defects BioCore Faculty. Gene-environment regulatory circuits of right ventricular pathology in Tetralogy of Fallot. J Mol Med. Ms. No. JMME-D-19-00563R1. In press
Touma M, Kang X, Gao F, Zhao Y, Cass AA, Biniwale R, Xiao X, Eghbali M, Coppola G, Reemtsen B, Wang Y (2017) Wnt11 regulates cardiac chamber development and disease during perinatal maturation. JCI Insight 2(17)
Finnemore A, Groves A (2015) Physiology of the fetal and transitional circulation. Semin Fetal Neonatal Med 20(4):210–216
Rudolph AM (2000) Myocardial growth before and after birth: clinical implications. Acta Paediatr 89(2):129–133
Sinha SK, Donn SM (2006) Fetal-to-neonatal maladaptation. Semin Fetal Neonatal Med 11(3):166–173
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA (2011) Transient regenerative potential of the neonatal mouse heart. Science. 331(6020):1078–1080
Touma M, Reemtsen B, Halnon N, Alejos J, Finn JP, Nelson SF, Wang Y (2017) A path to implement precision child health cardiovascular medicine. Front Cardiovasc Med 4:36
Pattersin AJ, Zhang L (2010) Hypoxia and fetal heart development. Curr Mol Med 10(7):653–666
Rohlicek CV, Matsuka T, Saiki C (2002) Cardiovascular response to acute hypoxemia in adult rats hypoxemic neonatally. Cardiovasc Res 53(1):263–270
Kimura W, Sadek HA (2012) The cardiac hypoxic niche: emerging role of hypoxic microenvironment in cardiac progenitors. Cardiovasc Diagn Ther 2(4):278–289
Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA (2017) Hypoxia induces heart regeneration in adult mice. Nature. 541(7636):222–227
Ziello JE, Jovin IS, Huang Y (2007) Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 80(2):51–60
Dyson HJ, Wright PE (2016) Role of intrinsic protein disorder in the function and interactions of the transcriptional coactivators CREB-binding protein (CBP) and p300. J Biol Chem 291(13):6714–6722
Yoon H, Lim JH, Cho CH, Huang LE, Park JW (2011) CITED2 controls the hypoxic signaling by snatching p300 from the two distinct activation domains of HIF-1α. Biochim Biophys Acta 1813(12):2008–2016
Von Gise A, Pu WT (2012) Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res 110(12):1628–1645
Sullivan KD, Galbraith MD, Andrysik Z, Espinosa JM (2018) Mechanisms of transcriptional regulation by p53. Cell Death Differ 25(1):133–143
Roder J, Linstid B, Oliveira C (2019) Improving the power of gene set enrichment analyses. BMC Bioinformatics 20(1):257
Peterson D, Lee J, Lei XC, Forrest WF, Davis DP, Jackson PK, Belmont LD (2010) A chemosensitization screen identifies TP53RK, a kinase that restrains apoptosis after mitotic stress. Cancer Res 70(15):6325–6335
Okamura S, Arakawa H, Tanaka T, Nakanishi H, Ng CC, Taya Y, Monden M, Nakamura Y (2001) p53DINP1, a p53-inducible gene, regulates p53-dependent apoptosis. Mol Cell 8(1):85–94
Sancho A, Duran J, García-España A, Mauvezin C, Alemu EA, Lamark T, Macias MJ, DeSalle R, Royo M, Sala D, Chicote JU, Palacín M, Johansen T, Zorzano A (2012) DOR/Tp53inp2 and Tp53inp1 constitute a metazoan gene family encoding dual regulators of autophagy and transcription. PLoS One 7(3):e34034
Fischer M, Steiner L, Engeland K (2014) The transcription factor p53: not a repressor, solely an activator. Cell Cycle 13(19):3037–3058
Sermeus A, Michiels C (2011) Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis 2:e164
Amelio I, Mancini M, Petrova V, Cairns RA, Vikhreva P, Nicolai S, Marini A, Antonov AA, Le Quesne J, Baena Acevedo JD, Dudek K, Sozzi G, Pastorino U, Knight RA, Mak TW, Melino G (2018) p53 mutants cooperate with HIF-1 in transcriptional regulation of extracellular matrix components to promote tumor progression. Proc Natl Acad Sci U S A 115(46):E10869–E10878
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Von Gise A, Zhou B, Honor LB, Ma Q, Petryk A, Pu WT (2011) WT1 regulates epicardial epithelial to mesenchymal transition through β-catenin and retinoic acid signaling pathways. Dev Biol 356(2):421–431
Cai X, Zhang W, Hu J, Zhang L, Sultana N, Wu B, Cai W, Zhou B, Cai CL (2013) Tbx20 acts upstream of Wnt signaling to regulate endocardial cushion formation and valve remodeling during mouse cardiogenesis. Development. 140(15):3176–3187
Jesse S, Koenig A, Ellenrieder V, Menke A (2010) Lef-1 isoforms regulate different target genes and reduce cellular adhesion. Int J Cancer 126(5):1109–1120
Blom JN, Feng Q (2018) Cardiac repair by epicardial EMT: current targets and a potential role for the primary cilium. Pharmacol Ther 186:114–129
Tsai YP, Wu KJ (2012) Hypoxia-regulated target genes implicated in tumor metastasis. J Biomed Sci 19:102
Li H, Rokavec M, Jiang L, Horst D, Hermeking H (2017) Antagonistic effects of p53 and HIF1A on microRNA-34a regulation of PPP1R11 and STAT3 and hypoxia-induced epithelial to Mesenchymal transition in colorectal cancer cells. Gastroenterology 153(2):505–520
Touma M, Kang X, Zhao Y, Cass AA, Gao F, Biniwale R, Coppola G, Xiao X, Reemtsen B, Wang Y (2016) Decoding the long noncoding RNA during cardiac maturation: a roadmap for functional discovery. Circ Cardiovasc Genet 9(5):395–407
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):RESEARCH0034
Luo W, Brouwer C (2013) Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 29(14):1830–1831
Acknowledgments
We acknowledge the support from UCLA Clinical Genomics Center, Animal Physiology Core, and the Congenital Heart Defects—BioCore at UCLA.
Funding
This work was supported by grants from the American Heart Association Career Development Award (18CDA34110414), the Department of Defense-Congressionally Directed Medical Research Programs (W81XWH-18-1-0164), the NIH/NHLBI (1R56HL146738-01), and the UCLA David Geffen School of Medicine Research Innovation Seed Grant to M. Touma.
Author information
Authors and Affiliations
Consortia
Contributions
MT conceived the project. MT, YZ, and XK designed and performed the research, analyzed most of the data, managed funding, and wrote the manuscript. AB, AJ, RB, GVA, NH, MG, and BR contributed to data acquisition and clinical insights. RPL and MW supported histology studies. FQR, WG, and SFN participated in manuscript review and editing.
Corresponding author
Ethics declarations
All animal-related experimental protocols were approved by the UCLA Institutional Animal Care and Use Committee (IACUC). Therefore, all studies have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The UCLA Congenital Heart Defect BioCore Faculty: Marlin Touma, Nancy Halnon, Brian Reemtsen, Juan Alejos, Reshma Biniwale, Myke Federman, Leigh Reardon, Meena Garg, Amy Speirs, John P. Finn, Fabiola Quintero-Rivera, Wayne Grody, Glen Van Arsdell, and Stanley Nelson.
Electronic supplementary material
ESM 1
(XLSX 27 kb)
Rights and permissions
About this article
Cite this article
Zhao, Y., Kang, X., Barsegian, A. et al. Gene-environment regulation of chamber-specific maturation during hypoxemic perinatal circulatory transition. J Mol Med 98, 1009–1020 (2020). https://doi.org/10.1007/s00109-020-01933-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01933-8