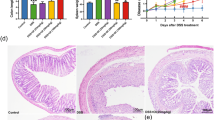
Abstract
Chromogranin-A (CHGA) is elevated in inflammatory bowel disease (IBD), but little is known about its role in colonic inflammation. IBD is associated with impaired functions of macrophages and increased apoptosis of intestinal epithelial cells. We investigated CHGA expression in human subjects with active ulcerative colitis (UC) and the underlying mechanisms in Chga −/− mice. In UC, CHGA, classically activated macrophage (M1) markers, caspase-3, p53, and its associated genes were increased, while alternatively activated macrophage (M2) markers were decreased without changes in the extrinsic apoptotic pathway. CHGA correlated positively with M1 and the apoptotic pathway and negatively with M2. In the murine dextran sulfate sodium (DSS)-induced colitis, Chga deletion reduced the disease severity and onset, pro-inflammatory mediators, M1, and p53/caspase-3 activation, while it upregulated anti-inflammatory cytokines and M2 markers with no changes in the extrinsic apoptotic markers. Compared to Chga +/+, M1 and p53/caspase-3 activation in Chga −/− macrophages were decreased in vitro, while M2 markers were increased. CHGA plays a critical role during colitis through the modulation of macrophage functions via the caspase-3/p53 pathway. Strategies targeting CHGA to regulate macrophage activation and apoptosis might be developed to treat UC patients.
Key messages
• Chromogranin-A (CHGA) is pro-hormone and is secreted in the gut. CHGA is elevated in colitis and is associated with the disease severity. The lack of GHGA has beneficial immunomodulatory properties during the development of intestinal inflammation. The lack of CHGA regulates the plasticity of macrophages and p53/caspase activation in colitis. Functional analysis of CHGA may lead to a novel therapy for IBD.







Similar content being viewed by others
References
D'amico MA, Ghinassi B, Izzicupo P, Manzoli L, Di Baldassarre A (2014) Biological function and clinical relevance of chromogranin A and derived peptides. Endocr Connect 3(2):R45–R54
Eissa N, Kermarrec L, Ghia J-E (2017) Neuroimmune mechanisms of cerebellar development and its developmental disorders: bidirectional link between the immune system and nervous system. In: Marzban H (ed) Development of the cerebellum from molecular aspects to diseases. Springer International Publishing, Cham, pp 255–274. https://doi.org/10.1007/978-3-319-59749-2_13
Eissa N, Hussein H, Kermarrec L, Elgazzar O, Metz-Boutigue MH, Bernstein CN, Ghia JE (2017) Chromofungin (CHR: CHGA47-66) is downregulated in persons with active ulcerative colitis and suppresses pro-inflammatory macrophage function through the inhibition of NF-kappaB signaling. Biochem Pharmacol 145:102–113
Eissa N, Hussein H, Kermarrec L, Grover J, Metz-Boutigue M-HE, Bernstein CN, Ghia J-E (2017) Chromofungin ameliorates the progression of colitis by regulating alternatively activated macrophages. Front Immunol 8:1131
Eissa N, Rabbi M, Bernstein C, Ghia J (2016) Chromofungin & pancreastatin co-regulate migration and functional plasticity of murine peritoneal macrophages. Neurogastroenterol Motil 28:103–104
Rabbi M, Eissa N, Munyaka P, Kermarrec L, Elgazzar O, Khafipour E, Bernstein C, Ghia J-E (2017) Reactivation of intestinal inflammation is suppressed by catestatin in a murine model of colitis via M1 macrophages and not the gut microbiota. Front Immunol 8:985
Rabbi MF, Labis B, Metz-Boutigue M-H, Bernstein CN, Ghia J-E (2014) Catestatin decreases macrophage function in two mouse models of experimental colitis. Biochem Pharmacol 89(3):386–398
Ahlman H, Nilsson O (2001) The gut as the largest endocrine organ in the body. Ann Oncol 12(suppl 2):S63–S68
Waldum HL, Syversen U (2000) Chromogranin A (CGA) and the enterochromaffin-like (ECL) cell. Adv Exp Med Bio, 482:361-367
Ghia J-E, Crenner F, Metz-Boutigue M-H, Aunis D, Angel F (2004) The effect of a chromogranin A-derived peptide (CgA4-16) in the writhing nociceptive response induced by acetic acid in rats. Life Sci 75(15):1787–1799
Sciola V, Massironi S, Conte D, Caprioli F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT, Piodi L (2009) Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm Bowel Dis 15(6):867–871
Zissimopoulos A, Vradelis S, Konialis M, Chadolias D, Bampali A, Constantinidis T, Efremidou E, Kouklakis G (2014) Chromogranin A as a biomarker of disease activity and biologic therapy in inflammatory bowel disease: a prospective observational study. Scand J Gastroenterol 49(8):942–949
Eissa N, Rabbi MF, Munyaka PM, Khafipour A, Bernstein CN, Ghia J-E (2016) Mo1929 critical role of chromogranin-A on macrophage intrinsic apoptotic pathway in colitis: human and animal studies. Gastroenterology 150(4):S819
El-Salhy M, Danielsson Å, Stenling R, Grimelius L (1997) Colonic endocrine cells in inflammatory bowel disease. J Intern Med 242(5):413–419
Lissner D, Schumann M, Batra A, Kredel L-I, Kühl AA, Erben U, May C, Schulzke J-D, Siegmund B (2015) Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis 21(6):1297–1305
Mosser DM, Zhang X (2008) Activation of murine macrophages. Current protocols in immunology / edited by John E Coligan et al CHAPTER: Unit. https://doi.org/10.1002/0471142735.im1402s83
Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK (1996) Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol 180(2):152–159
Man SM, Kanneganti T-D (2016) Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol 16(1):7–21
Marino G, Niso-Santano M, Baehrecke EH, Kroemer G (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15(2):81–94
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Spehlmann ME, Manthey CF, Dann SM, Hanson E, Sandhu SS, Liu LY, Abdelmalak FK, Diamanti MA, Retzlaff K, Scheller J (2013) Trp53 deficiency protects against acute intestinal inflammation. J Immunol 191(2):837–847
Sarnelli G, D’Alessandro A, Iuvone T, Capoccia E, Gigli S, Pesce M, Seguella L, Nobile N, Aprea G, Maione F (2016) Palmitoylethanolamide modulates inflammation-associated vascular endothelial growth factor (VEGF) signaling via the akt/mtor pathway in a selective peroxisome proliferator-activated receptor alpha (PPAR-α)-dependent manner. PLoS One 11(5):e0156198
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795
Hendy GN, Li T, Girard M, Feldstein RC, Mulay S, Desjardins R, Day R, Karaplis AC, Tremblay ML, Canaff L (2006) Targeted ablation of the chromogranin a (Chga) gene: normal neuroendocrine dense-core secretory granules and increased expression of other granins. Mol Endocrinol 20(8):1935–1947
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. (1990) A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98 (3):694-702
Cooper HS, Murthy S, Shah R, Sedergran D (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest J Tech Methods Pathol 69(2):238–249
Eissa N, Hussein H, Wang H, Rabbi MF, Bernstein CN, Ghia J-E (2016) Stability of reference genes for messenger RNA quantification by real-time PCR in mouse dextran sodium sulfate experimental colitis. PLoS One 11(5):e0156289
Eissa N, Kermarrec L, Hussein H, Bernstein CN, Ghia J-E (2017) Appropriateness of reference genes for normalizing messenger RNA in mouse 2, 4-dinitrobenzene sulfonic acid (DNBS)-induced colitis using quantitative real time PCR. Sci Rep 7:42427. https://doi.org/10.1038/srep42427
Eissa N, Hussein H, Rabbi MF, Munyaka PM, Khafipour A, Bernstein CN, Ghia J-E (2016) Tu1832 stability of reference genes for messenger RNA quantification by real-time PCR in mouse dextran sodium sulfate experimental colitis. Gastroenterology 150(4):S955–S956
Nie C, Luo Y, Zhao X, Luo N, Tong A, Liu X, Yuan Z, Wang C, Wei Y (2014) Caspase-9 mediates Puma activation in UCN-01-induced apoptosis. Cell Death Dis 5(10):e1495
Neurath MF (2014) Cytokines in inflammatory bowel disease. Nat Rev Immunol 14(5):329–342
Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M (2010) Chromogranin A—biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol 17(9):2427–2443
Di Comite G, Morganti A (2011) Chromogranin A: a novel factor acting at the cross road between the neuroendocrine and the cardiovascular systems. J Hypertens 29(3):409–414
Di Comite G, Marinosci A, Di Matteo P, Manfredi A, ROVERE-QUERINI P, Baldissera E, Aiello P, Corti A, Sabbadini MG (2006) Neuroendocrine modulation induced by selective blockade of TNF-α in rheumatoid arthritis. Ann N Y Acad Sci 1069(1):428–437
García S, Krausz S, Ambarus CA, Fernández BM, Hartkamp LM, van Es IE, Hamann J, Baeten DL, Tak PP, Reedquist KA (2014) Tie2 signaling cooperates with TNF to promote the pro-inflammatory activation of human macrophages independently of macrophage functional phenotype. PLoS One 9(1):e82088
Gottlieb PA, Delong T, Baker RL, Fitzgerald-Miller L, Wagner R, Cook G, Rewers MR, Michels A, Haskins K (2014) Chromogranin A is a T cell antigen in human type 1 diabetes. J Autoimmun 50:38–41
Steinbach EC, Plevy SE (2014) The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm Bowel Dis 20(1):166–175
Bandyopadhyay GK, Lu M, Avolio E, Siddiqui JA, Gayen JR, Wollam J, Vu CU, Chi N-W, O’Connor DT, Mahata SK (2015) Pancreastatin-dependent inflammatory signaling mediates obesity-induced insulin resistance. Diabetes 64(1):104–116
Jiménez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE (2016) The critical role of toll-like receptors—from microbial recognition to autoimmunity: a comprehensive review. Autoimmun Rev 15(1):1–8
Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ (2007) Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 133:1869–1881 e1814
Lin S, Li Y, Shen L, Zhang R, Yang L, Li M, Li K, Fichna J (2017) The anti-inflammatory effect and intestinal barrier protection of HU210 differentially depend on TLR4 signaling in dextran sulfate sodium-induced murine colitis. Dig Dis Sci 62(2):372–386
Goretsky T, Dirisina R, Sinh P, Mittal N, Managlia E, Williams DB, Posca D, Ryu H, Katzman RB, Barrett TA (2012) p53 mediates TNF-induced epithelial cell apoptosis in IBD. Am J Pathol 181(4):1306–1315
Arseneau KO, Tamagawa H, Pizarro TT, Cominelli F (2007) Innate and adaptive immune responses related to IBD pathogenesis. Curr Gastroenterol Rep 9(6):508–512
Parrish AB, Freel CD, Kornbluth S (2013) Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol 5(6):a008672
Shawgo ME, Shelton SN, Robertson JD (2008) Caspase-mediated Bak activation and cytochrome c release during intrinsic apoptotic cell death in Jurkat cells. J Biol Chem 283(51):35532–35538
Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ, Schoen RE, Yu J, Zhang L (2011) PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest 121(5):1722–1732
Parameswaran N, Patial S (2010) Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 20:87–103
van der Gracht E, Zahner S, Kronenberg M (2016) When insult is added to injury: cross talk between ILCs and intestinal epithelium in IBD. Mediat Inflamm 2016:9765238
Hofseth LJ, Si S, Hussain SP, Espey MG, Miranda KM, Araki Y, Jhappan C, Higashimoto Y, He P, Linke SP (2003) Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci 100(1):143–148
Rumio C, Dusio GF, Colombo B, Gasparri A, Cardani D, Marcucci F, Corti A (2012) The N-terminal fragment of chromogranin A, vasostatin-1 protects mice from acute or chronic colitis upon oral administration. Dig Dis Sci 57(5):1227–1237
Funding
This study was supported by grants from the Canadian Foundation for Innovation, Crohn’s and Colitis Canada, Research Manitoba, Children’s Hospital Research Institute of Manitoba, and the Canadian Institutes of Health Research to Jean-Eric Ghia. Nour Eissa is funded and supported by the Children’s Hospital Research Institute of Manitoba, Research Manitoba, University of Manitoba, Health Science Foundation-Mindel and Tom Olenick Research Excellence Award in Immunology, and MITACS accelerate program.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: NE and JEG. Performed the experiments: NE. Analyzed and interpreted the data: NE and JEG. Revised the data analysis and interpretation: NE, CNB, JEG. Performed research: HH, LK, AA, AM, MM, and GH. Contributed reagents/materials/analysis tools: JEG. Wrote the paper: NE, JEG. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
This study was approved by the University of Manitoba Health Research Ethics Board (HS14878 [E]). Persons undergoing colonoscopy either with known IBD or without IBD gave consent for the collection of biopsies. Experiments were approved by the University of Manitoba Animal Ethics Committee (Protocol No. 15-010) and were conducted under the Canadian guidelines for animal research.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 2621 kb).
Rights and permissions
About this article
Cite this article
Eissa, N., Hussein, H., Kermarrec, L. et al. Chromogranin-A Regulates Macrophage Function and the Apoptotic Pathway in Murine DSS colitis. J Mol Med 96, 183–198 (2018). https://doi.org/10.1007/s00109-017-1613-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1613-6