Abstract
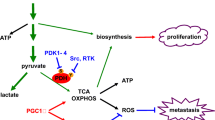
The shift by cancer cells toward aerobic glycolysis (Warburg effect) confers selective advantages by utilizing nutrients (e.g., lipids, amino acids, and nucleotides) to build biomass. Lipogenesis is generally enhanced, and its inhibition diminishes proliferation and survival. Re-expression of the metastasis suppressor KISS1 in human melanoma cells results in greater mitochondrial biogenesis, inhibition of glycolysis, utilization of beta-oxidation to provide energy, elevated oxidation of exogenous fatty acids, and increased expression of early-phase lipogenesis genes at both mRNA and protein levels. Correspondingly, the energy sensor AMPKβ is phosphorylated, resulting in inhibitory phosphorylation of acetyl-CoA carboxylase (ACC), which is linked to enhanced beta-oxidation. Furthermore, PGC1α is required for KISS1-mediated phosphorylation of ACC and metastasis suppression. Collectively, these data further support the linkages between macromolecular metabolism and metastasis.
Key messages
• KISS1 alters fatty acid metabolism.
• There may be connections between metastasis and metabolism.
• PGC1alpha appears to be downstream mediator of KISS1 metastasis suppression.








Similar content being viewed by others
References
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8:519–530
Crabtree HG (1928) The carbohydrate metabolism of certain pathological overgrowths. Biochem J 22:1289–1298
Crabtree HG (1929) Observations on the carbohydrate metabolism of tumours. Biochem J 23:536–545
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899
Kaelin WG, Thompson CB (2010) Q&A: cancer: clues from cell metabolism. Nature 465:562–564
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95
Dang CV (1999) c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol 19:1–11
Semenza GL (2009) Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol 19:12–16
Lopez-Rios F, Sanchez-Arago M, García-García E, Ortega AD, Berrendero JR, Pozo-Rodríguez F, Lopez-Encuentra A, Ballestín C, Cuezva JM (2007) Loss of the mitochondrial bioenergetic capacity underlies the glucose avidity of carcinomas. Cancer Res 67:9013–9017
Chiche J, Rouleau M, Gounon P, Brahimi-Horn MC, Pouyssegur J, Mazure NM (2010) Hypoxic enlarged mitochondria protect cancer cells from apoptotic stimuli. J Cell Physiol 222:648–657
Thompson CB (2009) Metabolic enzymes as oncogenes or tumor suppressors. N Engl J Med 360:813–815
Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320:661–664
Koshikawa N, Hayashi J, Nakagawara A, Takenaga K (2009) Reactive oxygen species-generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor-1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J Biol Chem 284:33185–33194
Ishikawa K, Hashizume O, Koshikawa N, Fukuda S, Nakada K, Takenaga K, Hayashi J (2008) Enhanced glycolysis induced by mtDNA mutations does not regulate metastasis. FEBS Lett 582:3525–3530
Pantel K, Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4:448–456
Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS (2011) Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer 11:135–141
Khanna C, Hunter K (2005) Modeling metastasis in vivo. Carcinogenesis 26:513–523
Eccles SA, Welch DR (2007) Metastasis: recent discoveries and novel treatment strategies. Lancet 369:1742–1757
Welch DR (2006) Defining a cancer metastasis. In: American Association for Cancer Research, ed. AACR Education Book 2006. Philadelphia: AACR, 111–115
Payen VL, Porporato PE, Baselet B, Sonveaux P (2016) Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci 73:1333–1348
Porporato PE, Payen VL, Baselet B, Sonveaux P (2016) Metabolic changes associated with tumor metastasis, part 2: mitochondria, lipid and amino acid metabolism. Cell Mol Life Sci 73:1349–1363
Lee J-H, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR (1996) KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 88:1731–1737
Lee J-H, Welch DR (1997) Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res 57:2384–2387
Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Torao Y, Kumano S, Takatsu Y, Matsuda Y et al (2001) Metastasis suppressor gene KiSS1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613–617
Harihar S, Pounds KM, Iwakuma T, Seidah NG, Welch DR (2014) Furin is the major proprotein convertase required for KISS1-to-Kisspeptin processing. PLoS One 9:e84958
Nash KT, Phadke PA, Navenot J-M, Hurst DR, Accavitti-Loper MA, Sztul E, Vaidya KS, Frost AR, Kappes JC, Peiper SC et al (2007) KISS1 metastasis suppressor secretion, multiple organ metastasis suppression, and maintenance of tumor dormancy. J Natl Cancer Inst 99:309–321
Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P et al (2001) AXOR12: a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 276:28969–28975
Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F et al (2001) The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636
Beck BH, Welch DR (2010) The KISS1 metastasis suppressor: a good night kiss for disseminated cancer cells. Eur J Cancer 46:1283–1289
Liu W, Beck BH, Vaidya KS, Nash KT, Feeley KP, Ballinger SW, Pounds KM, Denning WL, Diers AR, Landar A et al (2014) Metastasis suppressor KISS1 seems to reverse the Warburg effect by enhancing mitochondrial biogenesis. Cancer Res 74:954–963
Rogers GW, Nadanaciva S, Swiss R, Divakaruni AS, Will Y (2014) Assessment of fatty acid beta oxidation in cells and isolated mitochondria. Curr Protoc Toxicol 60:25–19
TeSlaa T, Teitell MA (2014) Techniques to monitor glycolysis. Methods Enzymol 542:91–114
Wilkins HM, Koppel S, Carl SM, Ramanujan S, Weidling I, Michaelis ML, Michaelis EK, Swerdlow RH (2016) Oxaloacetate enhances neuronal cell bioenergetic fluxes and infrastructure. J Neurochem 137:76–87
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Cheng G, Zielonka J, Dranka BP, McAllister D, Mackinnon AC Jr, Joseph J, Kalyanaraman B (2012) Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res 72:2634–2644
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z (2012) ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 14:1295–1304
Mathupala SP, Ko YH, Pedersen PL (2006) Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 25:4777–4786
Bartlett K, Eaton S (2004) Mitochondrial beta-oxidation. Eur J Biochem 271:462–469
Houten SM, Wanders RJ (2010) A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J Inherit Metab dis 33:469–477
Santos CR, Schulze A (2012) Lipid metabolism in cancer. FEBS J 279:2610–2623
Wang C, Rajput S, Watabe K, Liao DF, Cao D (2010) Acetyl-CoA carboxylase-a as a novel target for cancer therapy. Front Biosci (Schol Ed) 2:515–526
Hardie DG, Carling D (1997) The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur J Biochem 246:259–273
Warden SM, Richardson C, O'Donnell J Jr, Stapleton D, Kemp BE, Witters LA (2001) Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem J 354:275–283
Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A 101:3329–3335
Winder WW, Hardie DG (1996) Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Phys 270:E299–E304
Hardie DG, Corton J, Ching YP, Davies SP, Hawley S (1997) Regulation of lipid metabolism by the AMP-activated protein kinase. Biochem Soc Trans 25:1229–1231
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104:12017–12022
Tennant DA, Duran RV, Boulahbel H, Gottlieb E (2009) Metabolic transformation in cancer. Carcinogenesis 30:1269–1280
Simonnet H, Alazard N, Pfeiffer K, Gallou C, Beroud C, Demont J, Bouvier R, Schagger H, Godinot C (2002) Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis 23:759–768
Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Sotgia F et al (2012) Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal 16:1264–1284
Pastorino JG, Shulga N, Hoek JB (2002) Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 277:7610–7618
Navenot JM, Fujii N, Peiper SC (2009) KISS1 metastasis suppressor gene product induces suppression of tyrosine kinase receptor signaling to Akt, tumor necrosis factor family ligand expression, and apoptosis. Mol Pharmacol 75:1074–1083
Navenot JM, Fujii N, Peiper SC (2009) Activation of Rho and Rho-associated kinase by GPR54 and KiSS1 metastasis suppressor gene product induces changes of cell morphology and contributes to apoptosis. Mol Pharmacol 75:1300–1306
Lemasters JJ, Holmuhamedov E (2006) Voltage-dependent anion channel (VDAC) as mitochondrial governator—thinking outside the box. Biochim Biophys Acta 1762:181–190
Zhang F, Du G (2012) Dysregulated lipid metabolism in cancer. World J Biol Chem 3:167–174
Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr (2013) Cellular fatty acid metabolism and cancer. Cell Metab 18:153–161
Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP (1997) Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res 3:2115–2120
Swinnen JV, Vanderhoydonc F, Elgamal AA, Eelen M, Vercaeren I, Joniau S, Van PH, Baert L, Goossens K, Heyns W et al (2000) Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int J Cancer 88:176–179
Li W, Zhang C, Du H, Huang V, Sun B, Harris JP, Richardson Q, Shen X, Jin R, Li G et al (2016) Withaferin A suppresses the up-regulation of acetyl-coA carboxylase 1 and skin tumor formation in a skin carcinogenesis mouse model. Mol Carcinog 55:1739–1746
Yahagi N, Shimano H, Hasegawa K, Ohashi K, Matsuzaka T, Najima Y, Sekiya M, Tomita S, Okazaki H, Tamura Y et al (2005) Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer 41:1316–1322
Wu X, Daniels G, Lee P, Monaco ME (2014) Lipid metabolism in prostate cancer. Am J Clin Exp Urol 2:111–120
Blum R, Kloog Y (2014) Metabolism addiction in pancreatic cancer. Cell Death Dis 5:e1065
Rodrigues MF, Obre E, de Melo FH, Santos GC Jr, Galina A, Jasiulionis MG, Rossignol R, Rumjanek FD, Amoedo ND (2016) Enhanced OXPHOS, glutaminolysis and beta-oxidation constitute the metastatic phenotype of melanoma cells. Biochem J 473:703–715
Carracedo A, Cantley LC, Pandolfi PP (2013) Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 13:227–232
Shackelford DB, Shaw RJ (2009) The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9:563–575
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141
Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, Allegra M, Giacchero D, Bahadoran P, Bertolotto C et al (2013) Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther 12:1605–1615
Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI (2002) AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A 99:15983–15987
Rohas LM, St-Pierre J, Uldry M, Jager S, Handschin C, Spiegelman BM (2007) A fundamental system of cellular energy homeostasis regulated by PGC-1alpha. Proc Natl Acad Sci U S A 104:7933–7938
Luo C, Lim JH, Lee Y, Granter SR, Thomas A, Vazquez F, Widlund HR, Puigserver P (2016) A PGC1alpha-mediated transcriptional axis suppresses melanoma metastasis. Nature 537:422–426
Acknowledgements
This study was supported by grants to DRW from Susan G. Komen for the Cure [SAC11037], the National Foundation for Cancer Research-Center for Metastasis Research, US National Cancer Institute RO1-CA134981, and using partial support from RO1-CA87728 and P30-CA168524. D.R. Welch is the Hall Family Professor of Molecular Medicine and a Kansas Bioscience Authority Eminent Scholar. This study also was supported by fellowship to SJM from the Kansas INBRE, P20 GM103418.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Manley, S.J., Liu, W. & Welch, D.R. The KISS1 metastasis suppressor appears to reverse the Warburg effect by shifting from glycolysis to mitochondrial beta-oxidation. J Mol Med 95, 951–963 (2017). https://doi.org/10.1007/s00109-017-1552-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1552-2