Abstract
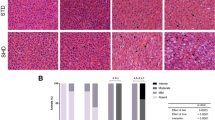
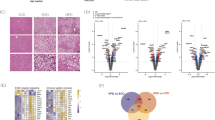
Non-alcoholic fatty liver disease (NAFLD) is a metabolic liver disease that is thought to be reversible by changing the diet. To examine the impact of dietary changes on progression and cure of NAFLD, we fed mice a high-fat diet (HFD) or high-fructose diet (HFrD) for 9 weeks, followed by an additional 9 weeks, where mice were given normal chow diet. As predicted, the diet-induced NAFLD elicited changes in glucose tolerance, serum cholesterol, and triglyceride levels in both diet groups. Moreover, the diet-induced NAFLD phenotype was reversed, as measured by the recovery of glucose intolerance and high cholesterol levels when mice were given normal chow diet. However, surprisingly, the elevated serum triglyceride levels persisted. Metagenomic analysis revealed dietary-induced changes of microbiome composition, some of which remained altered even after reversing the diet to normal chow, as illustrated by species of the Odoribacter genus. Genome-wide DNA methylation analysis revealed a “priming effect” through changes in DNA methylation in key liver genes. For example, the lipid-regulating gene Apoa4 remained hypomethylated in both groups even after introduction to normal chow diet. Our results support that dietary change, in part, reverses the NAFLD phenotype. However, some diet-induced effects remain, such as changes in microbiome composition, elevated serum triglyceride levels, and hypomethylation of key liver genes. While the results are correlative in nature, it is tempting to speculate that the dietary-induced changes in microbiome composition may in part contribute to the persistent epigenetic modifications in the liver.





Similar content being viewed by others
References
Fatemeh H, Elham F, Peyman A (2017) Nonalcoholic fatty liver disease: diagnostic biomarkers. World J Gastrointest Pathophysiol 8:11–26
Romero-Gomez MZ-S, Trenell SM (2017) Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 67:829–846
Haufe S, Hass V, Utz W, Birkenfeld AL, Jeran S, Bohnke J, Mahler A, Luft FC, Schulz-Menger J, Boschmann M, Jordan J, Engeli S (2013) Long-lasting improvements in liver fat and metabolism despite body weight regain after dietary weight loss. Diabetes Care 36:3786–3792
Pugh CJA, Spring VS, Jones H, Richardson P, Shojaee-Moradies F, Umpleby AM, Green DJ, Cable NT, Trenell MI, Kemp GJ, Cuthbertson DJ (2016) Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int J Obes (Lond) 40:1927–1930
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S (2012) Host-gut microbiota metabolic interactions. Science 336:1262–1267
Kundu P, Blacher E, Elinav E, Pettersson S (2017) Our gut microbiome: the evolving inner self. Cell 171:1481–1493
Leung C, Rivera L, Furness JB, Angus PW (2016) The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol 13:412–425
Backhed F, Manchester JK, Semenkovich CF, Gordon JI (2007) Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. PNAS. 104:979–984
Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, Bailey J, Myers RP, Rioux KP (2013) Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 11(868–75):e1–e3
Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR (2013) Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 57:601–609
Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L (2018) Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 67:1881–1891
Kumar H, Lund R, Laiho A, Lundelin K, Ley RE, Isolauri E, Salminen S (2014) Gut microbiota as an epigenetic regulator: pilot study based on whole-genome methylation analysis. MBio 5:e02113–e02114
Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, Keller MP, Attie AD, Rey FE, Denu JM (2016) Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell 64:982–992
Sookoian S, Rosselli MS, Gemma C, Burgueno AL, Fernandez Gianotti T, Castano GO, Pirola CJ (2010) Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor gamma coactivator 1alpha promoter. Hepatology 52:1992–2000
Del Campo JA, Gallego-Duran R, Gallego P, Grande L (2018) Genetic and epigenetic regulation in nonalcoholic fatty liver disease (NAFLD). Int J Mol Sci 19:911–921
Murphy SK, Yang H, Moylan CA, Pang H, Dellinger A, Abdelmalek MF, Garrett ME, Ashley-Koch A, Suzuki A, Tillmann HL, Hauser MA, Diehl AM (2013) Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology 145:1076–1087
Pogribny IP, Tryndyak VP, Bagnyukova TV, Melnyk S, Montgomery B, Ross SA, Latendresse JR, Rusyn I, Beland FA (2009) Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol 51:176–186
Gg H (1991) Multipoint dixon technique for water and fat proton and susceptibility imaging. J Magn Reson Imaging 1:521–530
Fiebig T, Boll H, Figueiredo G, Kerl HU, Nittka S, Groden C, Kramer M, Brockmann MA (2012) Three-dimensional in vivo imaging of the murine liver: a micro-computed tomography-based anatomical study. PLoS One 7:e31179
Gabriel A, Kukla M, Ziokowki A (2008) Histopathological features and current scoring systmes for semiquantitative assessment of nonalcoholic fatty liver disease. Exp Clin Hepatol 4:48–54
Gu H, Bock C, Mikkelsen TS, Jager N, Smith ZD, Tomazou E, Gnirke A, Lander ES, Meissner A (2010) Genome-scale DNA methylation mapping of clinical samples at single-nucleotide resolution. Nat Methods 7:133–136
Tillman EJ, Morgan DA, Rahmouni K, Swoap SJ (2014) Three months of high-fructose feeding fails to induce excessive weight gain or leptin resistance in mice. PLoS One 9:e107206
Clarke SF, Murphy EF, O’Sullivan O, Ross RP, O’Toole PW, Shanahan F, Cotter PD (2013) Targeting the microbiota to address diet-induced obesity: a time dependent challenge. PLoS One 8:e65790
Fukui H (2015) Gut microbiota and host reaction in liver diseases. Microorganisms 3:759–791
Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, Reo NV (2015) Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol 91:1–9
Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L (2014) Alterations of the human gut microbiome in liver cirrhosis. Nature 513:59–64
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60
Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YY, Young JD, Lai HC (2015) Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun 6:7489
Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M (2016) Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 68:974–981
Subramanian S, Goodspeed L, Wang S, Kim J, Zeng L, Ioannou GN, Haigh WG, Yeh MM, Kowdley KV, O’Brien KD, Pennathur S, Chait A (2011) Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res 52:1626–1635
Yoon A, Tammen SA, Park S, Han SN, Choi S-W (2017) Genome-wide hepatic DNA methylation changes in high-fat diet-induced obese mice. Nutr Res Pract 11:105–113
Zhou D, Hlady RA, Schafer MJ, White TA, Liu C, Choi J-H, Miller JD, Roberts LR, LeBrasseur NK, Robertson KD (2017) High fat diet and exercise lead to a disrupted and pathogenic DNA methylome in mouse liver. Epigenetics. 12:55–69
Jo J-C, Choi EK, Shin J-S, Moon J-H, Hong S-W, Lee H-R, Kim S-M, Jung S-A, Lee D-H, Jung SH, Lee S-H, Kim JE, K-p Kim, Hong YS, Suh Y-A, Jang SJ, Choi EK, Lee JS, Jin D-H, Kim TW (2015) Targeting FGFR pathway in human hepatocellular carcinoma: expressing pFGFR and pMET for antitumor activity. Mol Cancer Ther 14:2613–2622
Cheng AL, Shen YC, Zhu AX (2011) Targeting fibroblast growth factor receptor signaling in hepatocellular carcinoma. Oncology 81:372–380
Wang J, Li J, Wang X, Zheng C, Ma W (2013) Downregulation of microRNA-214 and overexpression of FGFR-1 contribute to hepatocellular carcinoma metastasis. Biochem Biophys Res Commun 439:47–53
Laguna JC, Alegret M, Roglans N (2014) Simple sugar intake and hepatocellular carcinoma: epidemiological and mechanistic insight. Nutrients 6:5933–5954
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16:6–21
Suzuki MM, Bird A (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 9:465–476
Park EY, Choi H, Yoon JY, Lee IY, Seo Y, Moon HS, Hwang JH, Jun HS (2015) Polyphenol-rich fraction of Ecklonia cava improves nonalcoholic fatty liver disease in high fat diet-fed mice. Mar Drugs 13:6866–6883
Ushio M, Nishio Y, Sekine O, Nagai Y, Maeno Y, Ugi S, Yoshizaki T, Morino K, Kume S, Kashiwagi A, Maegawa H (2013) Ezetimibe prevents hepatic steatosis induced by a high-fat but not a high-fructose diet. Am J Physiol Endocrinol Metab 305:E293–E304
Bocarsly ME, Powell ES, Avena NM, Hoebel BG (2010) High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav 97:101–106
Thaiss CA, Itav S, Rothschild D, Meijer M, Levy M, Moresi C, Dohnalova L, Braverman S, Rozin S, Malitsky S, Dori-Bachash M, Kuperman Y, Biton I, Gertler A, Harmelin A, Shapiro H, Halpern Z, Aharoni A, Segal E, Elinav E (2016) Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 540:544–551
Deaton AM, Bird A (2011) CpG islands and the regulation of transcription. Genes Dev 25:1010–1022
Wang F, Kohan AB, Lo CM, Liu M, Howles P, Tso P (2015) Apolipoprotein A-IV: a protein intimately involved in metabolism. J Lipid Res 56:1403–1418
VerHague MA, Cheng D, Weinberg RB, Shelness GS (2013) Apolipoprotein A-IV expression in mouse liver enhances triglyceride secretion and reduces hepatic lipid content by promoting very low density lipoprotein particle expansion. Arterioscler Thromb Vasc Biol 33:2501–2508
Acknowledgements
We thank our colleagues, Alicia Kang, Llanto Elma Faylon, Kok Huan Teo, and Norhashimah Binte Sulaiman from Nanyang Technological University for their assistance in animal maintaining and data collections that greatly contributed the manuscript. We thank Li Yiqing from National University Health System for his assistance with animal experiments. We thank Saraf Sahil, Loh Jie Hua, and Sam Xin Xiu from Singapore General Hospital for their great help in steatosis scoring. We also thank Zenia Tiang from the Genome Institute of Singapore with her help in library submission for sequencing. This work was supported by the LKC School of Medicine Start Up Grant, MOE TIER 1 Grant, Grant from SCELSE and EU Grant TORNADO awarded to Sven Pettersson. This work also was supported a CSIRC award (National Medical Research Council Singapore) and a SPF Grant (Biomedical Research Council Singapore) awarded to Roger Foo.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2019_3114_MOESM2_ESM.pdf
Supplementary material 2 (PDF 393 kb) Figure S2. Daily food intakes for each diet. (A) Amount of daily food intake per mouse for 15 weeks. Food intake measured 2 times/week for 24 h and makes average for 1 week. (B) Daily caloric intake. *P < 0.05 for NC vs. HFD, two-way ANOVA (C) Liver weight, normalized to the tibia length (TA). *P < 0.05 for NC vs. HFD, one-way ANOVA
18_2019_3114_MOESM3_ESM.pdf
Supplementary material 3 (PDF 436 kb) Figure S3. IPGTT in mice show diet effects on glucose tolerance. Blood glucose levels were measured during IPGTTs, administered after (A) test diets and (B) reversal diets *P < 0.05 for NC vs. HFD, # < 0.05 for NC vs. HFrD, two-way ANOVA. IL18 (C) TLR4 (D) gene expression measured with quantitative RT-PCR, and normalized to housekeeping gene expression levels. * P≤0.05 for NC vs. HFD or HFrD, R_NC vs. R_HFD, one-way ANOVA
18_2019_3114_MOESM4_ESM.pdf
Supplementary material 4 (PDF 10945 kb) Figure S4. Changes in gut microbiome induced by diet changes. (A) Bubble chart shows top 30 bacterial genera from each diet group. Bubble sizes represent the relative size of gut bacteria populations. Bar graphs show changes in (B) Prevotella *P < 0.05 NC vs. HFD or HFrD; #P < 0.05 HFD vs. R_HFD; and HFrD vs. R_HFrD; one-way ANOVA; (C) Akkermansia *P < 0.05 NC vs. HFD or HFrD; #P < 0.05 HFD vs. R_HFD; and HFrD vs. R_HFrD, one-way ANOVA; and (D) Parabacteroides goldsteinii *P < 0.05 NC vs. HFrD; #P < 0.05 R_HFD vs. R_NC or R_HFrD. (E) Normalized abundance of butyrate kinase in gut microbiome; #P < 0.05 R-HFD vs. R_NC or R_HFrD
18_2019_3114_MOESM5_ESM.pdf
Supplementary material 5 (PDF 475 kb) Figure S5. Sites of differential methylation in DNA from mice fed HFD and HFrD diets. (A) Pie charts show the distribution of sites, where significant dmCpGs are found in DNA, during test diets and after returning to NC. UTR = untranslated region. (B) Traces show the average DNA methylation levels around the TSS and TES regions, where all significant dmCpGs are found, TSS = transcription start site, TES = transcription end site. dmCpG significance level was P≤0.05
18_2019_3114_MOESM6_ESM.pdf
Supplementary material 6 (PDF 386 kb) Figure S6. Heat map shows changes in gene expression levels for enzymes critical in DNA methylation, for each diet. Expression was measured with quantitative RT-PCR, normalized to housekeeping gene expression levels, and respective controls (NC and R_NC). Red indicates minimal change in gene expression; blue is a ~ twofold increase in gene expression
18_2019_3114_MOESM7_ESM.pdf
Supplementary material 7 (PDF 681 kb) Figure S7. (A) Representative western blot of APOA4 in the liver (n = 2) (B) Relative intensity of APOA4 in the liver (n = 8-10 mice per group). Levels were normalized with corresponding β-actin levels
Rights and permissions
About this article
Cite this article
Kim, H., Worsley, O., Yang, E. et al. Persistent changes in liver methylation and microbiome composition following reversal of diet-induced non-alcoholic-fatty liver disease. Cell. Mol. Life Sci. 76, 4341–4354 (2019). https://doi.org/10.1007/s00018-019-03114-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03114-4