Abstract
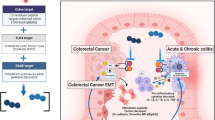
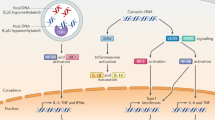
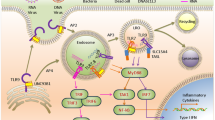
Colorectal cancer (CRC) is a leading cause of cancer-related deaths that is often associated with inflammation initiated by activation of pattern recognition receptors (PRRs). Nucleic acid sensing PRRs are one of the major subsets of PRRs that sense nucleic acid (DNA and RNA), mainly including some members of Toll-like receptors (TLR3, 7, 8, 9), AIM2-like receptors (AIM2, IFI16), STING, cGAS, RNA polymerase III, and DExD/H box nucleic acid helicases (such as RIG-I like receptors (RIG-I, MDA5, LPG2), DDX1, 3, 5, 7, 17, 21, 41, 60, and DHX9, 36). Activation of these receptors eventually leads to the release of cytokines and activation of immune cells, which are well known to play crucial roles in host defense against intracellular bacterial and virus infection. However, the functions of these nucleic acid sensing PRRs in the other diseases such as CRC and colitis remain largely unknown. Recent studies indicated that nucleic acid sensing PRRs contribute to CRC and/or colitis development, and therapeutic modulation of nucleic acid sensing PRRs may reduce the risk of CRC development. However, until now, a comprehensive review on the role of nucleic acid sensing PRRs in CRC and colitis is still lacking. This review provided an overview of the roles as well as the mechanisms of these nucleic acid sensing PRRs (AIM2, STING, cGAS, RIG-I and its downstream molecules, DDX3, 5, 6,17, and DHX9, 36) in CRC and colitis, which may aid the diagnosis, therapy, and prognostic prediction of CRC and colitis.




Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2016) Global patterns and trends in colorectal cancer incidence and mortality. Gut. doi:10.1136/gutjnl-2015-310912
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA-Cancer J Clin 66:115–132
Brenner H, Kloor M, Pox CP (2014) Colorectal cancer. The Lancet 383:1490–1502
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820
Meylan E, Tschopp J, Karin M (2006) Intracellular pattern recognition receptors in the host response. Nature 442:39–44
Parlato M, Yeretssian G (2014) NOD-like receptors in intestinal homeostasis and epithelial tissue repair. Int J Mol Sci 15:9594–9627
Nunes T, de Souza HS (2013) Inflammasome in intestinal inflammation and cancer. Mediators Inflamm 2013:654963
Li TT, Ogino S, Qian ZR (2014) Toll-like receptor signaling in colorectal cancer: carcinogenesis to cancer therapy. World J Gastroenterol 20:17699–17708
Gurtler C, Bowie AG (2013) Innate immune detection of microbial nucleic acids. Trends Microbiol 21:413–420
Paludan SR, Bowie AG (2013) Immune sensing of DNA. Immunity 38:870–880
Man SM, Karki R, Kanneganti TD (2016) AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol 46:269–280
Chen GY (2014) Role of Nlrp6 and Nlrp12 in the maintenance of intestinal homeostasis. Eur J Immunol 44:321–327
So EY, Ouchi T (2010) The application of Toll like receptors for cancer therapy. Int J Biol Sci 6:675–681
Slattery ML, Herrick JS, Bondurant KL, Wolff RK (2012) Toll-like receptor genes and their association with colon and rectal cancer development and prognosis. Int J Cancer 130:2974–2980
Furi I, Sipos F, Germann TM, Kalmar A, Tulassay Z, Molnar B, Muzes G (2013) Epithelial toll-like receptor 9 signaling in colorectal inflammation and cancer: clinico-pathogenic aspects. World J Gastroenterol 19:4119–4126
Sipos F, Furi I, Constantinovits M, Tulassay Z, Muzes G (2014) Contribution of TLR signaling to the pathogenesis of colitis-associated cancer in inflammatory bowel disease. World J Gastroenterol 20:12713–12721
Ratsimandresy RA, Dorfleutner A, Stehlik C (2013) An update on PYRIN domain-containing pattern recognition receptors: from immunity to pathology. Front Immunol 4:440
Hornung V, Ablasser A, Charrel Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514–518
Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS (2010) IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11:997–1004
Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B (2011) IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 9:363–375
Zaki MH, Lamkanfi M, Kanneganti TD (2011) The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol 32:171–179
Hu B, Jin C, Li HB, Tong J, Ouyang X, Cetinbas NM, Zhu S, Strowig T, Lam FC, Zhao C, Henao-Mejia J, Yilmaz O, Fitzgerald KA, Eisenbarth SC, Elinav E, Flavell RA (2016) The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 354:765–768
DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, Trapani JA, Meltzer PS, Trent JM (1997) Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 15:453–455
Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, Panchanathan R, Choubey D (2013) AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res 11:1193–1202
Dihlmann S, Tao S, Echterdiek F, Herpel E, Jansen L, Chang-Claude J, Brenner H, Hoffmeister M, Kloor M (2014) Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer 135:2387–2396
Chen LC, Wang LJ, Tsang NM, Ojcius DM, Chen CC, OuYang CN (2012) Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol Med 12:1276–1293
Poulogiannis G, Frayling IM, Arends MJ (2010) DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology 56:167–179
Woerner SM, Kloor M, Schwitalle Y, Youmans H, Doeberitz MvK, Gebert J, Dihlmann S (2007) The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Gene Chromosome Canc 46:1080–1089
Kim TM, Laird PW, Park PJ (2013) The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell 155:858–868
Guo H, Callaway JB, Ting JP (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687
Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP-Y (2010) The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. Int J Clin Exp Med 207:1045–1056
Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, Eisenbarth SC, Flavell RA (2010) Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Nat Immunol 107:21635–21640
Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti T-D (2010) IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol 185:4912–4920
Hu B, Elinav E, Flavell RA (2011) Inflammasome-mediated suppression of inflammation-induced colorectal cancer progression is mediated by direct regulation of epithelial cell proliferation. Cell Cycle 10:1936–1939
Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, Muhlbauer M, Chou WC, Barker BR, Jobin C, Allbritton NL, Ramsden DA, Davis BK, Ting JP (2015) Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med 21:906–913
Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P, Neale G, Olsen SR, Carter RA, McGoldrick DJ, Wu G, Finkelstein D, Vogel P, Gilbertson RJ, Kanneganti TD (2015) Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell 162:45–58
Snider AJ, Bialkowska AB, Ghaleb AM, Yang VW, Obeid LM, Hannun YA (2016) Murine model for colitis-associated cancer of the colon. Methods Mol Biol 1438:245–254
Feng J, Park J, Cron P, Hess D, Hemmings BA (2004) Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem 279:41189–41196
Hresko RC, Mueckler M (2005) mTOR· RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem 280:40406–40416
Van der Flier LG, Clevers H (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H (2009) Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457:608–611
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW (1997) Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275:1787–1790
Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP (2010) Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 12:468–476
Reya T, Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434:843–850
Patsos G, Germann A, Gebert J, Dihlmann S (2010) Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer 126:1838–1849
Hu S, Peng L, KwakYT, Tekippe EM, Pasare C, Malter JS, Hooper LV, Zaki MH (2015) The DNA sensor AIM2 maintains intestinal homeostasis via regulation of epithelial antimicrobial host defense. Cell Rep 13:1922–1936
Hu G, Song P, Li N, Chen W, Lei Q, Yu S, Zhang X, Du C, Deng X, Han W (2016) AIM2 contributes to the maintenance of intestinal integrity via Akt and protects against Salmonella mucosal infection. Mucosal Immuno l9:1330–1339
Ratsimandresy R A, Indramohan M, Dorfleutner A, Stehlik C (2016) The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell Mol Immunol. doi:10.1038/cmi.2016.35
Pikor L, Thu K, Vucic E, Lam W (2013) The detection and implication of genome instability in cancer. Cancer Metastasis Rev 32:341–352
Burdette D L, Monroe K M, Sotelo-Troha K, Iwig J S, Eckert B, Hyodo M, Hayakawa Y, Vance R E (2011) STING is a direct innate immune sensor of cyclic di-GMP. Nature 478:515–518
Schaap P (2013) Cyclic di-nucleotide signaling enters the eukaryote domain. Lubmb Life 65:897–903
Ishikawa , Ma Z, Barber G N (2009) STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–792
Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Tanigui T (2007) DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501–505
Zhang Z, Yuan B, Bao M, LuN, KimT, LiuYJ (2011) The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol 12:959–965
Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791
Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Röhl I, Hopfner KP, Ludwig J, Hornung V (2013) cGAS produces a 2 [prime]-5 [prime]-linked cyclic dinucleotide second messenger that activates STING. Nature 498:380–384
Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ (2013) Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell 51:226–235
Chen H, Sun H, You F, Sun W, Zhou X, Chen L, Yang J, Wang Y, Tang H, Guan Y (2011) Activation of STAT6 by STING is critical for antiviral innate immunity. Cell 147:436–446
Zhu Q, Man SM, Gurung P, Liu Z, Vogel P, Lamkanfi M, Kanneganti TD (2014) Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J Immunol 193:4779–4782
Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufman R, Huber LA, Zatloukal K (2005) Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia 7:545–555
Ahn J, Konno H, Barber G (2015) Diverse roles of STING-dependent signaling on the development of cancer. Oncogene 34:5302–5308
Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, HuB, Hedl M, Zhang W, O’Connor W, Murphy AJ (2012) IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491:259–263
Dumoutier L, Lejeune D, Colau D, Renauld JC (2001) Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol 166:7090–7095
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H (1998) CD8 + T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF (2011) Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8+ dendritic cells. J Exp Med 208:2005–2016
Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA (2014) STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41:830–842
Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T (2014) STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41:843–852
Bode C, Fox M, Tewary P, Steinhagen A, Ellerkmann RK, Klinman D, Baumgarten G, Hornung V, Steinhagen F (2016) Human plasmacytoid dentritic cells elicit a Type I Interferon response by sensing DNA via the cGAS-STING signaling pathway. Eur J Immunol 46:1615–1621
Andzinski L, Spanier J, Kasnitz N, Kroger A, Jin L, Brinkmann MM, Kalinke U, Weiss S, Jablonska J, Lienenklaus S (2016) Growing tumors induce a local STING dependent Type I IFN response in dendritic cells. Int J Cancer 139:1350–1357
Xia T, Konno H, Ahn J, Barber GN (2016) Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep 14:282–297
Peedicayil J (2012) The role of DNA methylation in the pathogenesis and treatment of cancer. Curr Clin Pharmacol 7:333–340
Reinert LS, Lopusna K, Winther H, Sun C, Thomsen MK, Nandakumar R, Mogensen TH, Meyer M, Vaegter C, Nyengaard JR, Fitzgerald KA, Paludan SR (2016) Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat Commun 7:13348
Zhang L, Tatsuya T, Nishiyama Y (2016) Oncotarget Strategies For Herpes Simplex Virus-1. Curr Gene Ther 16:130–143
Xia T, Konno H, Barber GN (2016) Recurrent loss of STING signaling in melanoma correlates with susceptibility to viral oncolysis. Cancer Res76: 6747-6759
Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DK, Gravekamp C (2014) STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res 2:901–910
Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ (2015) Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 11:1018–1030
Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W (2015) STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 7:283ra52–283rara52
Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, Munn D, Mellor AL (2016) STING Promotes the Growth of Tumors Characterized by Low Antigenicity via IDO Activation. Cancer Res 76:2076–2081
Ding L, Huang XF, Dong GJ, Hu EL, Sheng C, Wang TT, Hu QG, Yan-HongN, Ni YH (2015) Activated STING enhances Tregs infiltration in the HPV-related carcinogenesis of tongue squamous cells via the c-jun/CCL22 signal. Biochim Biophys Acta 1852:2494–2503
Tanner NK, Linder P (2001) DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol Cell 8:251–262
Loo YM, Gale M (2011) Immune signaling by RIG-I-like receptors. Immunity 34:680–692
Schmidt A, Schwerd T, Hamm W, Hellmuth JC, Cui S, Wenzel M, Hoffmann FS, Michallet MC, Besch R, Hopfner KP (2009) 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc Natl Acad Sci USA 106:12067–12072
Hornung V (2014) SnapShot: nucleic acid immune sensors, part 2. Immunity 41:1066–1067
Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu R, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reise Sousa C, Matsuura Y, Fujita T, Akira S (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105
Broquet AH, Hirata Y, McAllister CS, Kagnoff MF (2011) RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J Immunol 186:1618–1626
Errett JS, Suthar MS, McMillan A, Diamond MS, Gale MJ (2013) The essential, nonredundant roles of RIG-I and MDA5 in detecting and controlling West Nile virus infection. J Viro l87:11416–11425
Wang Y, Zhang HX, Sun YP, Liu ZX, Liu XS, Wang L, Lu SY, Kong H, Liu QL, Li XH (2007) Rig-I–/– mice develop colitis associated with downregulation of Gαi2. Cell Res 17:858–868
Wang Y, Zhang HX, Yue PS, Liu X (2011) Regression of Peyer’s patches in G alpha i2 deficient mice prior to colitis is associated with reduced expression of Bcl-2 and increased apoptosis. Cell Res 17:858–868
Arinze IJ, Kawai Y (2005) Transcriptional activation of the human Galphai2 gene promoter through nuclear factor-kappaB and antioxidant response elements. J Biol Chem 280:9786–9795
Funke B, Lasitschka F, Roth W, Penzel R, Meuer S, Saile M, Gretz N, Sido B, Schirmacher P, Autschbach F (2011) Selective downregulation of retinoic acid-inducible gene I within the intestinal epithelial compartment in crohn’s disease. Inflamm Bowel Dis 17:1943–1954
Li XD, Chiu YH, Ismail AS, Behrendt CL, Wight-Carter M, Hooper LV, Chen ZJ (2011) Mitochondrial antiviral signaling protein (MAVS) monitors commensal bacteria and induces an immune response that prevents experimental colitis. Proc Natl Acad Sci USA 108:17390–17395
Negishi H, Miki S, Sarashina H, Taguchi-Atarashi N, Nakajima A, Matsuki K, Endo N, Yanai H, Nishio J, Honda K (2012) Essential contribution of IRF3 to intestinal homeostasis and microbiota-mediated Tslp gene induction. P Natl Acad Sci USA 109:21016–21021
Ryan CW, Parekha AD, Rancka MC, Goldena DW, Kumara KA, Sooda RF, Pitrodaa SP, Liaoa Z, Huanga X, Dargaa TE, Xua D, Huangb L, Andradeb J (2014) RIG-I–like receptor LGP2 protects tumor cells from ionizing radiation. Proc Natl Acad Sci USA 111:E484–E491
Fullam A, Schröder M (2013) DExD/H-box RNA helicases as mediators of anti-viral innate immunity and essential host factors for viral replication. BBA-Gene Regul Mec 1829:854–865
Moy RH, Cole BS, Yasunaga A, Gold B, Shankarling G, Varble A, Molleston JM, tenOever BR, Lynch KW, Cherry S (2014) Stem-loop recognition by DDX17 facilitates miRNA processing and antiviral defense. Cell 158:764–777
Chen G, Liu CH, Zhou L, Krug RM (2014) Cellular DDX21 RNA helicase inhibits influenza A virus replication but is counteracted by the viral NS1 protein. Cell Host Microbe 15:484–493
Yu SF, Lujan P, Jackson DL, Emerman M, Linial ML (2011) The DEAD-box RNA helicase DDX6 is required for efficient encapsidation of a retroviral genome. PLoS Pathog 7:e1002303
Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, Liu ZJ, Modlin RL, Liu YJ, Cheng G (2012) The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol 13:1155–1161
Kadono M, Kanai A, Nagamachi A, Shinriki S, Kawata J, Iwato K, Kyo T, Oshima K, Yokoyama A, Kawamura T, Nagase R, Inoue D, Kitamura T, Inaba T, Ichinohe T, Matsui H (2016) Biological implications of somatic DDX41 p.R525H mutation in acute myeloid leukemia. Exp Hematol 44:745–54e4
Cardoso SR, Ryan G, Walne AJ, Ellison A, Lowe R, Tummala H, Rio-Machin A, Collopy L, Al Seraihi A, Wallis Y, Page P, Akiki S, Fitzgibbon J, Vulliamy T, Dokal I (2016) Germline heterozygous DDX41 variants in a subset of familial myelodysplasia and acute myeloid leukemia. Leukemia 30:2083–2086
Li R, Sobreira N, Witmer PD, Pratz KW, Braunstein EM (2016) Two novel germline DDX41 mutations in a family with inherited myelodysplasia/acute myeloid leukemia. Haematologica 101:e228–e231
Lewinsohn M, Brown AL, Weinel LM, Phung C, Rafidi G, Lee MK, Schreiber AW, Feng J, Babic M, Chong CE, Lee Y, Yong A, Suthers GK, Poplawski N, Altree M, Phillips K, Jaensch L, Fine M, D’Andrea RJ, Lewis ID, Medeiros BC, Pollyea DA, King MC, Walsh T, Keel S, Shimamura A, Godley LA, Hahn CN, Churpek JE, Scott HS (2016) Novel germ line DDX41 mutations define families with a lower age of MDS/AML onset and lymphoid malignancies. Blood 127:1017–1023
Berger G, van denBerg E, Sikkema-Raddatz B, Abbott KM, Sinke RJ, Bungener LB, Mulder AB, Vellenga E (2016) Re-emergence of acute myeloid leukemia in donor cells following allogeneic transplantation in a family with a germline DDX41 mutation. Leukemia 31(2):520–522
Ditton HJ, Zimmer J, Kamp C, Rajpert-De Meyts E (2004) The AZFa gene DBY (DDX3Y) is widely transcribed but the protein is limited to the male germ cells by translation control. Hum Mol Genet 13:2333–2341
Zhan T, Rindtorff N, Boutros M (2016) Wnt signaling in cancer. Oncogene. doi:10.1038/onc.2016.304
Cruciat C-M, Dolde C, Groot REAd, Ohkawara B, Carmen Reinhard1 HCK, Christof Niehrs (2013) RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in Wnt-β-catenin signaling. Science 339:1436–1441
Gurzu S, Silveanu C, Fetyko A, Butiurca V, Kovacs Z, Jung I (2016) Systematic review of the old and new concepts in the epithelial-mesenchymal transition of colorectal cancer. World J Gastroenterol 22:6764–6775
Botlagunta M, Vesuna F, Mironchik Y, Raman A, Lisok A, Winnard P Jr, Mukadam S, VanDiest P, Chen JH, Farabaugh P, Patel AH, Raman V (2008) Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene 27:3912–3922
Su CY, Lin TC, Lin YF, Chen MH (2015) DDX3 as a strongest prognosis marker and its downregulation promotes metastasis in colorectal cancer. Oncotarget 6:18602–18612
Heerma van Voss MR, Vesuna F, Trumpi K, Brilliant J, Berlinicke C, de Leng W, Kranenburg O, Offerhaus GJ, Bürger H, van der Wall E, van Diest PJ, Raman V (2015) Identification of the DEAD box RNA helicase DDX3 as a therapeutic target in colorectal cancer. Oncotarget 6:28312–28326
Wu DW, Lin PL, Cheng YW, Huang CC, Wang L, Lee H (2016) DDX3 enhances oncogenic KRAS induced tumor invasion in colorectal cancer via the betacatenin/ZEB1 axis. Oncotarget 7:22687–22699
He TY, Wu DW, Lin PL, Wang L, Huang CC, Chou MC, Lee H (2016) DDX3 promotes tumor invasion in colorectal cancer via the CK1epsilon/Dvl2 axis. Sci Rep. doi:10.1038/srep21483
Brai A, Fazi R, Tintori C, Zamperini C, Sanguinetti M (2016) Human DDX3 protein is a valuable target to develop broad spectrum antiviral agents. Proc Natl Acad Sci USA 113:5388–5393
Zhang S, Grosse F (1994) Nuclear DNA helicase II unwinds both DNA and RNA. Biochem 33:3906–3912
Jain A, Bacolla A, Del Mundo IM, Zhao J, Wang G, Vasquez KM (2013) DHX9 helicase is involved in preventing genomic instability induced by alternatively structured DNA in human cells. Nucleic Acids Res 41:10345–10357
Wei X, Pacyna-Gengelbach M, Schluns K, An Q, Gao Y, Cheng S, Petersen I (2004) Analysis of the RNA helicase A gene in human lung cancer. Oncol Rep11:253–258
Sun Z, Wang L, Eckloff BW, Deng B, Wang Y (2014) Conserved recurrent gene mutations correlate with pathway deregulation and clinical outcomes of lung adenocarcinoma in never-smokers. BMC Med Genomics 7:32. doi:10.1186/1755-8794-7-32
Mills JR, Malina A, Lee T, Man SM (2013) RNAi screening uncovers Dhx9 as a modifier of ABT-737 resistance in an Eμ-myc/Bcl-2 mouse model. Blood 121:3402–3412
Lee T, Di Paola D, Malina A, Mills JR, Kreps A, Grosse F, Tang H, Zannis-Hadjopoulos M, Larsson O, Pelletier J (2014) Suppression of the DHX9 helicase induces premature senescence in human diploid fibroblasts in a p53-dependent manner. J Biol Chem 289:22798–22814
Fidaleo M, Svetoni F, Volpe E, Minana B, Caporossi D, Paronetto MP (2015) Genotoxic stress inhibits Ewing sarcoma cell growth by modulating alternative pre-mRNA processing of the RNA helicase DHX9. Oncotarget 6:31740–31757
Albrethsen J, Knol JC, Piersma S, VT (2010) Sub-nuclear proteomics in colorectal cancer: Identification of proteins enriched in the nuclear matrix fraction and regulation in adenoma to carcinoma progression. Mol Cell Proteomics 9:988–1005
Mil J, Ray P, Liu J, Kuan C-T (2016) In Vivo selection against human colorectal cancer xenografts identifies an aptamer that targets RNA helicase protein DHX9. MolTher-Nucl Acids 5:1–9
Fuller-PaceFV (2013) DEAD box RNA helicase functions in cancer. RNA Biol 10:121–132
Fuller-Pace FV, Ali S (2008) The DEAD box RNA helicases p68 (Ddx5) and p72 (Ddx17): novel transcriptional co-regulators. Biochem Soc Trans 36:609–612
Shin S, Rossow KL, Grande JP, Janknecht R (2007) Involvement of RNA helicases p68 and p72 in colon cancer. Cancer Res 67:7572–7578
Causevic M, Hislop RG, Kernohan NM, Carey FA, Kay RA, Steele RJ, Fuller-Pace FV (2001) Overexpression and poly-ubiquitylation of the DEAD-box RNA helicase p68 in colorectal tumours. Oncogene 20:7734–7743
Nakagawa Y, Morikawa H, Hirata I, Shiozaki M, Matsumoto A, Maemura K, Nishikawa T, Niki M, Tanigawa N, Ikegami M, Katsu K, Akao Y (1999) Overexpression of rck/p54, a DEAD box protein, in human colorectal tumours. Br J Cancer 80:914–917
Lin F, Wang R, Shen JJ, Wang X, Gao P, Dong K, Zhang HZ (2008) Knockdown of RCK/p54 expression by RNAi inhibits proliferation of human colorectal cancer cells in vitro and in vivo. Cancer Biol Ther 7:1669–1676
Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA, Qin J, Cheng G, Liu YJ (2011) DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity 34:866–878
Fu JJ, Li LY, Lu GX (2002) Molecular cloning and characterization of human DDX36 and mouse Ddx36 genes, new members of the DEAD/H box superfamily. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 34:655–661
Matsumura K, Kawasaki Y, Miyamoto M, Kamoshida Y, Nakamura J, Negishi L, Suda S, Akiyama T (2016) The novel G-quadruplex-containing long non-coding RNA GSEC antagonizes DHX36 and modulates colon cancer cell migration. Oncogene. doi:10.1038/onc.2016.282
Acknowledgements
This work was supported by funds from Talents’ Start-up Fund of Gannan Medical University (QD201404), Natural Science Foundation of Jiangxi Province (20151BAB205061), Natural Science Foundation of China (31560260), and The Key Project from Department of Education of Jiangxi Province (150937) (All to Zhiping Liu).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, L., Chen, Y., Wu, Y. et al. Nucleic acid sensing pattern recognition receptors in the development of colorectal cancer and colitis. Cell. Mol. Life Sci. 74, 2395–2411 (2017). https://doi.org/10.1007/s00018-017-2477-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2477-1