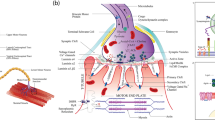
Abstract
The neuromuscular junction (NMJ) is the most extensively studied model of neuronal synaptogenesis. Acetylcholine receptor (AChR) clustering on the postsynaptic membrane is a cardinal event in the differentiation of NMJs. AChR clustering and postsynaptic differentiation is orchestrated by sophisticated interactions among three proteins: the neuron-secreted proteoglycan agrin, the co-receptor LRP4, and the muscle-specific receptor tyrosine kinase MuSK. LRP4 and MuSK act as scaffolds for multiple binding partners, resulting in a complex and dynamic network of interacting proteins that is required for AChR clustering. In this review, we discuss the structural basis for NMJ postsynaptic differentiation mediated by the agrin–LRP4–MuSK signaling pathway.




Similar content being viewed by others
Abbreviations
- AChR:
-
Acetylcholine receptor
- Fz-CRD:
-
Frizzled cysteine-rich domain
- Ig-like:
-
Immunoglobulin-like
- LG:
-
Laminin globular
- LRP4:
-
Low-density lipoprotein receptor-related protein 4
- MuSK:
-
Muscle-specific kinase
- NMJ:
-
Neuromuscular junction
- NtA:
-
N-terminal agrin
- PH:
-
Pleckstrin homology
- PTB:
-
Phosphotyrosine binding
- RTK:
-
Receptor tyrosine kinase
- Tid1:
-
Tumorous imaginal disc 1
References
Slater CR (2008) Reliability of neuromuscular transmission and how it is maintained. Handb Clin Neurol 91:27–101
Madhavan R, Peng HB (2005) Molecular regulation of postsynaptic differentiation at the neuromuscular junction. IUBMB Life 57:719–730
Sanes JR, Lichtman JW (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci 2:791–805
Fertuck HC, Salpeter MM (1976) Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol 69:144–158
Burden SJ (2002) Building the vertebrate neuromuscular synapse. J Neurobiol 53:501–511
Ferraro E, Molinari F, Berghella L (2012) Molecular control of neuromuscular junction development. J Cachexia Sarcopenia Muscle 3:13–23
Wu H, Xiong WC, Mei L (2010) To build a synapse: signaling pathways in neuromuscular junction assembly. Development 137:1017–1033
Witzemann V (2006) Development of the neuromuscular junction. Cell Tissue Res 326:263–271
Ngo ST, Noakes PG, Phillips WD (2007) Neural agrin: a synaptic stabiliser. Int J Biochem Cell Biol 39:863–867
Sigoillot SM, Bourgeois F, Lambergeon M, Strochlic L, Legay C (2010) ColQ controls postsynaptic differentiation at the neuromuscular junction. J Neurosci 30:13–23
Amenta AR et al (2012) Biglycan is an extracellular MuSK binding protein important for synapse stability. J Neurosci 32:2324–2334
Singhal N, Martin PT (2011) Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev Neurobiol 71:982–1005
Ghazanfari N, Fernandez KJ, Murata Y, Morsch M, Ngo ST, Reddel SW, Noakes PG, Phillips WD (2011) Muscle specific kinase: organiser of synaptic membrane domains. Int J Biochem Cell Biol 43:295–298
Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L (2008) LRP4 serves as a coreceptor of agrin. Neuron 60:285–297
Kim N et al (2008) Lrp4 is a receptor for agrin and forms a complex with MuSK. Cell 135:334–342
Weatherbee SD, Anderson KV, Niswander LA (2006) LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development 133:4993–5000
Song Y, Balice-Gordon R (2008) New dogs in the dogma: lrp4 and Tid1 in neuromuscular synapse formation. Neuron 60:526–528
Antolik C, Catino DH, Resneck WG, Bloch RJ (2006) The tetratricopeptide repeat domains of rapsyn bind directly to cytoplasmic sequences of the muscle-specific kinase. Neuroscience 141:87–100
McMahan UJ (1990) The agrin hypothesis. Cold Spring Harb Symp Quant Biol 55:407–418
Nitkin RM, Smith MA, Magill C, Fallon JR, Yao YM, Wallace BG, McMahan UJ (1987) Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J Cell Biol 105:2471–2478
Bezakova G, Ruegg MA (2003) New insights into the roles of agrin. Nat Rev Mol Cell Biol 4:295–308
Ferns MJ, Campanelli JT, Hoch W, Scheller RH, Hall Z (1993) The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron 11:491–502
Hoch W, Campanelli JT, Scheller RH (1994) Structural domains of agrin required for clustering of nicotinic acetylcholine receptors. EMBO J 13:2814–2821
McMahan UJ, Horton SE, Werle MJ, Honig LS, Kroger S, Ruegg MA, Escher G (1992) Agrin isoforms and their role in synaptogenesis. Curr Opin Cell Biol 4:869–874
Cohen NA, Kaufmann WE, Worley PF, Rupp F (1997) Expression of agrin in the developing and adult rat brain. Neuroscience 76:581–596
Kroger S, Mann S (1996) Biochemical and functional characterization of basal lamina-bound agrin in the chick central nervous system. Eur J Neurosci 8:500–509
Ksiazek I et al (2007) Synapse loss in cortex of agrin-deficient mice after genetic rescue of perinatal death. J Neurosci 27:7183–7195
McFarlane AA, Stetefeld J (2009) An interdomain disulfide bridge links the NtA and first FS domain in agrin. Protein Sci 18:2421–2428
Mascarenhas JB, Ruegg MA, Sasaki T, Eble JA, Engel J, Stetefeld J (2005) Structure and laminin-binding specificity of the NtA domain expressed in eukaryotic cells. Matrix Biol 23:507–513
Stetefeld J et al (2001) The laminin-binding domain of agrin is structurally related to N-TIMP-1. Nat Struct Biol 8:705–709
Caterina NC, Windsor LJ, Yermovsky AE, Bodden MK, Taylor KB, Birkedal-Hansen H, Engler JA (1997) Replacement of conserved cysteines in human tissue inhibitor of metalloproteinases-1. J Biol Chem 272:32141–32149
Huang W, Meng Q, Suzuki K, Nagase H, Brew K (1997) Mutational study of the amino-terminal domain of human tissue inhibitor of metalloproteinases 1 (TIMP-1) locates an inhibitory region for matrix metalloproteinases. J Biol Chem 272:22086–22091
Mascarenhas JB, Ruegg MA, Winzen U, Halfter W, Engel J, Stetefeld J (2003) Mapping of the laminin-binding site of the N-terminal agrin domain (NtA). EMBO J 22:529–536
Cornish T, Chi J, Johnson S, Lu Y, Campanelli JT (1999) Globular domains of agrin are functional units that collaborate to induce acetylcholine receptor clustering. J Cell Sci 112(Pt 8):1213–1223
Gesemann M, Denzer AJ, Ruegg MA (1995) Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J Cell Biol 128:625–636
Tseng CN, Zhang L, Wu SL, Wang WF, Wang ZZ, Cascio M (2010) Asparagine of z8 insert is critical for the affinity, conformation, and acetylcholine receptor-clustering activity of neural agrin. J Biol Chem 285:27641–27651
Tseng CN, Zhang L, Cascio M, Wang ZZ (2003) Calcium plays a critical role in determining the acetylcholine receptor-clustering activities of alternatively spliced isoforms of Agrin. J Biol Chem 278:17236–17245
Tidow H, Mattle D, Nissen P (2011) Structural and biophysical characterisation of agrin laminin G3 domain constructs. Protein Eng Des Sel 24:219–224
Stetefeld J et al (2004) Modulation of agrin function by alternative splicing and Ca2+ binding. Structure 12:503–515
Rudenko G, Hohenester E, Muller YA (2001) LG/LNS domains: multiple functions—one business end? Trends Biochem Sci 26:363–368
Godfrey EW, Roe J, Heathcote RD (2000) Agrin fragments differentially induce ectopic aggregation of acetylcholine receptors in myotomal muscles of Xenopus embryos. J Neurobiol 44:436–445
Ruegg MA, Tsim KW, Horton SE, Kroger S, Escher G, Gensch EM, McMahan UJ (1992) The agrin gene codes for a family of basal lamina proteins that differ in function and distribution. Neuron 8:691–699
Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E (2000) Structure and function of laminin LG modules. Matrix Biol 19:309–317
O’Toole JJ, Deyst KA, Bowe MA, Nastuk MA, McKechnie BA, Fallon JR (1996) Alternative splicing of agrin regulates its binding to heparin alpha-dystroglycan, and the cell surface. Proc Natl Acad Sci USA 93:7369–7374
Campanelli JT, Gayer GG, Scheller RH (1996) Alternative RNA splicing that determines agrin activity regulates binding to heparin and alpha-dystroglycan. Development 122:1663–1672
Maselli RA et al (2011) LG2 agrin mutation causing severe congenital myasthenic syndrome mimics functional characteristics of non-neural (z-) agrin. Hum Genet 131(7):1123–1135
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134
Hopf C, Hoch W (1998) Dimerization of the muscle-specific kinase induces tyrosine phosphorylation of acetylcholine receptors and their aggregation on the surface of myotubes. J Biol Chem 273:6467–6473
Xie MH, Yuan J, Adams C, Gurney A (1997) Direct demonstration of MuSK involvement in acetylcholine receptor clustering through identification of agonist ScFv. Nat Biotechnol 15:768–771
Glass DJ et al (1996) Agrin acts via a MuSK receptor complex. Cell 85:513–523
DeChiara TM et al (1996) The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85:501–512
Till JH, Becerra M, Watty A, Lu Y, Ma Y, Neubert TA, Burden SJ, Hubbard SR (2002) Crystal structure of the MuSK tyrosine kinase: insights into receptor autoregulation. Structure 10:1187–1196
Johnson LN, Noble ME, Owen DJ (1996) Active and inactive protein kinases: structural basis for regulation. Cell 85:149–158
Songyang Z, Margolis B, Chaudhuri M, Shoelson SE, Cantley LC (1995) The phosphotyrosine interaction domain of SHC recognizes tyrosine-phosphorylated NPXY motif. J Biol Chem 270:14863–14866
Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL (2005) Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol 345:1–20
Jones G, Moore C, Hashemolhosseini S, Brenner HR (1999) Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J Neurosci 19:3376–3383
Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, Birchmeier C, Burden SJ (2001) Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30:399–410
Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF (2001) Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410:1057–1064
Grassot J, Gouy M, Perriere G, Mouchiroud G (2006) Origin and molecular evolution of receptor tyrosine kinases with immunoglobulin-like domains. Mol Biol Evol 23:1232–1241
Zhou H, Glass DJ, Yancopoulos GD, Sanes JR (1999) Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. J Cell Biol 146:1133–1146
Wang Q, Zhang B, Wang YE, Xiong WC, Mei L (2008) The Ig1/2 domain of MuSK binds to muscle surface and is involved in acetylcholine receptor clustering. Neurosignals 16:246–253
Stiegler AL, Burden SJ, Hubbard SR (2006) Crystal structure of the agrin-responsive immunoglobulin-like domains 1 and 2 of the receptor tyrosine kinase MuSK. J Mol Biol 364:424–433
Ultsch MH, Wiesmann C, Simmons LC, Henrich J, Yang M, Reilly D, Bass SH, de Vos AM (1999) Crystal structures of the neurotrophin-binding domain of TrkA, TrkB and TrkC. J Mol Biol 290:149–159
Jennings CG, Dyer SM, Burden SJ (1993) Muscle-specific trk-related receptor with a kringle domain defines a distinct class of receptor tyrosine kinases. Proc Natl Acad Sci USA 90:2895–2899
Masiakowski P, Yancopoulos GD (1998) The Wnt receptor CRD domain is also found in MuSK and related orphan receptor tyrosine kinases. Curr Biol 8:R407
Xu YK, Nusse R (1998) The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr Biol 8:R405–R406
Jing L, Lefebvre JL, Gordon LR, Granato M (2009) Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron 61:721–733
Banerjee S, Gordon L, Donn TM, Berti C, Moens CB, Burden SJ, Granato M (2011) A novel role for MuSK and non-canonical Wnt signaling during segmental neural crest cell migration. Development 138:3287–3296
Strochlic L et al (2012) Wnt4 participates in the formation of vertebrate neuromuscular junction. PLoS One 7:e29976
Zhang B, Liang C, Bates R, Yin Y, Xiong WC, Mei L (2012) Wnt proteins regulate acetylcholine receptor clustering in muscle cells. Mol Brain 5:7
Stiegler AL, Burden SJ, Hubbard SR (2009) Crystal structure of the frizzled-like cysteine-rich domain of the receptor tyrosine kinase MuSK. J Mol Biol 393:1–9
Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ (2001) Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412:86–90
Carron C, Pascal A, Djiane A, Boucaut JC, Shi DL, Umbhauer M (2003) Frizzled receptor dimerization is sufficient to activate the Wnt/beta-catenin pathway. J Cell Sci 116:2541–2550
Oishi I et al (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8:645–654
Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC (2012) Structural basis of Wnt recognition by Frizzled. Science 337:59–64
May P, Woldt E, Matz RL, Boucher P (2007) The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med 39:219–228
Hussain MM (2001) Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front Biosci 6:D417–D428
Go GW, Mani A (2012) Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J Biol Med 85:19–28
Willnow TE, Nykjaer A, Herz J (1999) Lipoprotein receptors: new roles for ancient proteins. Nat Cell Biol 1:E157–E162
Simon-Chazottes D, Tutois S, Kuehn M, Evans M, Bourgade F, Cook S, Davisson MT, Guenet JL (2006) Mutations in the gene encoding the low-density lipoprotein receptor LRP4 cause abnormal limb development in the mouse. Genomics 87:673–677
Johnson EB, Steffen DJ, Lynch KW, Herz J (2006) Defective splicing of Megf7/Lrp4, a regulator of distal limb development, in autosomal recessive mulefoot disease. Genomics 88:600–609
Zong Y et al (2012) Structural basis of agrin-LRP4-MuSK signaling. Genes Dev 26:247–258
Bourhis E et al (2011) Wnt antagonists bind through a short peptide to the first beta-propeller domain of LRP5/6. Structure 19:1433–1442
Takagi J, Yang Y, Liu JH, Wang JH, Springer TA (2003) Complex between nidogen and laminin fragments reveals a paradigmatic beta-propeller interface. Nature 424:969–974
Choi HY, Dieckmann M, Herz J, Niemeier A (2009) Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One 4:e7930
Leupin O et al (2011) Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem 286:19489–19500
Zhang W, Coldefy AS, Hubbard SR, Burden SJ (2011) Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK). J Biol Chem 286:40624–40630
Gomez AM, Burden SJ (2011) The extracellular region of Lrp4 is sufficient to mediate neuromuscular synapse formation. Dev Dyn 240:2626–2633
Cai D, Dhe-Paganon S, Melendez PA, Lee J, Shoelson SE (2003) Two new substrates in insulin signaling, IRS5/DOK4 and IRS6/DOK5. J Biol Chem 278:25323–25330
Mashima R, Hishida Y, Tezuka T, Yamanashi Y (2009) The roles of Dok family adapters in immunoreceptor signaling. Immunol Rev 232:273–285
Inoue A, Setoguchi K, Matsubara Y, Okada K, Sato N, Iwakura Y, Higuchi O, Yamanashi Y (2009) Dok-7 activates the muscle receptor kinase MuSK and shapes synapse formation. Sci Signal 2(59):ra7
Okada K et al (2006) The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science 312:1802–1805
Hamuro J et al (2008) Mutations causing DOK7 congenital myasthenia ablate functional motifs in Dok-7. J Biol Chem 283:5518–5524
Beeson D et al (2006) Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science 313:1975–1978
Bergamin E, Hallock PT, Burden SJ, Hubbard SR (2010) The cytoplasmic adaptor protein Dok7 activates the receptor tyrosine kinase MuSK via dimerization. Mol Cell 39:100–109
Dhe-Paganon S, Ottinger EA, Nolte RT, Eck MJ, Shoelson SE (1999) Crystal structure of the pleckstrin homology-phosphotyrosine binding (PH-PTB) targeting region of insulin receptor substrate 1. Proc Natl Acad Sci USA 96:8378–8383
Linnoila J, Wang Y, Yao Y, Wang ZZ (2008) A mammalian homolog of Drosophila tumorous imaginal discs, Tid1, mediates agrin signaling at the neuromuscular junction. Neuron 60:625–641
Gaestel M (2006) Molecular chaperones in signal transduction. Handb Exp Pharmacol 172:93–109
Lu B, Garrido N, Spelbrink JN, Suzuki CK (2006) Tid1 isoforms are mitochondrial DnaJ-like chaperones with unique carboxyl termini that determine cytosolic fate. J Biol Chem 281:13150–13158
Qiu XB, Shao YM, Miao S, Wang L (2006) The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci 63:2560–2570
Hennessy F, Boshoff A, Blatch GL (2005) Rational mutagenesis of a 40 kDa heat shock protein from Agrobacterium tumefaciens identifies amino acid residues critical to its in vivo function. Int J Biochem Cell Biol 37:177–191
Tsai J, Douglas MG (1996) A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding. J Biol Chem 271:9347–9354
Syken J, De-Medina T, Munger K (1999) TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions. Proc Natl Acad Sci USA 96:8499–8504
Lee Y, Rudell J, Ferns M (2009) Rapsyn interacts with the muscle acetylcholine receptor via alpha-helical domains in the alpha, beta, and epsilon subunit intracellular loops. Neuroscience 163:222–232
Moransard M, Borges LS, Willmann R, Marangi PA, Brenner HR, Ferns MJ, Fuhrer C (2003) Agrin regulates rapsyn interaction with surface acetylcholine receptors, and this underlies cytoskeletal anchoring and clustering. J Biol Chem 278:7350–7359
Ramarao MK, Bianchetta MJ, Lanken J, Cohen JB (2001) Role of rapsyn tetratricopeptide repeat and coiled-coil domains in self-association and nicotinic acetylcholine receptor clustering. J Biol Chem 276:7475–7483
Acknowledgments
We apologize to our colleagues whose work could not be individually cited due to space limitations. R.J. acknowledges support from the Alfred P. Sloan Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zong, Y., Jin, R. Structural mechanisms of the agrin–LRP4–MuSK signaling pathway in neuromuscular junction differentiation. Cell. Mol. Life Sci. 70, 3077–3088 (2013). https://doi.org/10.1007/s00018-012-1209-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1209-9