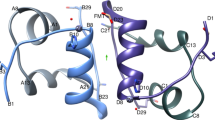
Abstract
Using the crystal structure of Despentapeptide (B26-B30) insulin (DPI as the search model, the crystal structure of DesB1-B2 Despentapeptide (B26-B30) insulin (DesB1-2 DPI) has been studied by the molecular replacement method. There is one DesB1-2 DPI molecule in each crystallographic asymmetric unit. The cross rotation function search and the translation function search show apparent peaks and thus determine the orientation and position of DesB1-2 DPI molecule in the cell respectively. The subsequent three-dimensional structural rebuilding and refinement of DesB1-2 DPI molecule confirm the results by molecular replacement method.
Similar content being viewed by others
References
Liang, D. C., Chang, W. R., Wang, D. C.et al., The characteristics and motion model of insulin monomer,Science in China, Ser. B, 1992, 35(4): 418.
Liang, D. C., Chang, W. R., Zhang, G. P.et al., The possible mechanism of binding interaction of insulin molecule with its receptorScience in China, Ser. B, 1992, 35(5): 547.
Liang, D.C., Chang, W.R., Wan, Z.L., A proposed interaction model of the insulin molecule with its receptor,Biophys, Chem., 1994, 50(1,2): 63.
Liang, D. C., Studies of the three-dimensional structure of insulin and its analogs, inBeijing Biomacromolecule Laboratory, 1988, Beijing: Institute of Biophysics, 113–150.
Chu, J. H., Chu, S. C., Chang, Y. S., Effects of shortening of B chain N terminus and C terminus on the antibody-binding ability of insulin,Acta Biochimica et Biophisica Sinicu, 1984, 16(3): 264.
Schwartz, G., Katsoyannis, P.G., Synthesis of Des (tetrapeptide B1-4) and Des (pentapeptide B1-4) human insulins,Biochemistry, 1978, 17(21): 4550.
Lei, K.J., Dong, B., Xie, D.L.et al., Studies of insulin’s analogs shortened at B chain N terminus (II): the circular dichroism and biological and immunological activity of desB1-3 insulin,Acta Biochimica et Biophisica Sinica, 1983, 15(5): 457.
Wan, Z.L., Diao, J.S., Chang, W.R.et al., Preliminary crystallographic studies of DesB1-2 Despentapeptide (B26-30) insulin,Chinese Science Bulletin (in Chinese), 1994, 39(12): 1130.
Matthews, B.W., Solvent content of protein crystals,J. Mol. Biol., 1968, 33(10): 491.
Brunger, A.T.,X-PLOR Manual, A System for Crystallography and NMR, Version 3. 1, New Haven: Yale University Press, 1992.
Dai, J.B., Lou, M.Z., You, J.M.et al., Refinement of the structure of Despentapeptide (B26-30) at 1. 5Å resolution,Science in China, Ser. B, 1987, 30(1): 54.
Author information
Authors and Affiliations
Additional information
Project supported by the Foundation of Chinese Academy of Sciences and the National Natural Science Foundation of China.
Rights and permissions
About this article
Cite this article
Diao, J., Wan, Z., Chang, W. et al. Molecular replacement studies on crystal structure of DesB1-B2 Despentapeptide (B26-B30) insulin. Sci. China Ser. C.-Life Sci. 40, 524–530 (1997). https://doi.org/10.1007/BF03183592
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03183592