Abstract
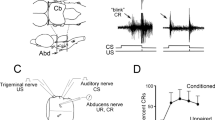
Exposure of the developing brain to alcohol produces profound Purkinje cell loss in the cerebellum, and deficits in tests of motor coordination. However, the precise relationship between these two sets of findings has been difficult to determine. Eyeblink classical conditioning is known to engage a discrete brainstem-cerebellar circuit, making it an ideal test of cerebellar functional integrity after developmental alcohol exposure. In eyeblink conditioning, one of the deep cerebellar nuclei, the interpositus nucleus, as well as specific Purkinje cell populations, are sites of convergence for CS and US information. A series of studies have shown that eyeblink conditioning is impaired in both weanling and adult rats given binge-like exposure to alcohol as neonates, and that these deficits can be traced, at least in part, to impaired activation of cerebellar interpositus nucleus neurons and to an overall reduction in the deep cerebellar nuclear cell population. Because particular cerebellar cell populations are utilized in well-defined ways during eyeblink conditioning, conclusions regarding specific changes in the mediation of behavior by these cell populations are greatly strengthened. Further studies will be directed towards the impact of early exposure to alcohol on the functionality of specific Purkinje cell populations, as well as towards brainstem areas that process the tone CS and the somatosensory US.
Similar content being viewed by others
References
Albus, J. S. (1971). A theory of cerebellar function.Mathematical Biosciences, 10, 25–61.
Altman, J. (1969). Autoradiographic and histological studies of postnatal neurogenesis. III. Dating the time of production and onset of differentiation of cerebellar microneurons in rats.Journal of Comparative Neurology, 136, 269–294.
Altman, J. (1972a). Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer.Journal of Comparative Neurology, 145, 353–398.
Altman, J. (1972b). Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer.Journal of Comparative Neurology, 145, 399–464.
Altman, J. (1972c). Postnatal development of the cerebellar cortex in the rat. III. Maturation of the components of the granular layer.Journal of Comparative Neurology, 145, 465–514.
Altman, J., & Bayer, S. A. (1985a). Embryonic development of the rat cerebellum. II. Translocation and regional distribution of the deep neurons.Journal of Comparative Neurology, 231, 27–41.
Altman, J., & Bayer, S. A. (1985b). Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells.Journal of Comparative Neurology, 231, 42–65.
Andrews, S. J., Freeman, J. H., Carter, C. S., & Stanton, M. E. (1995). Ontogeny of eyeblink conditioning in the rat: Auditory frequency and discrimination learning effects.Developmental Psychobiology, 28, 307–320.
Attwell, P. J. E., Cooke, S. F., & Yeo, C. H. (2002). Cerebellar function in consolidation of a motor memory.Neuron, 34, 1011–1020.
Attwell, P. J. E., Rahman, S., Ivarsson, M., & Yeo, C. H. (1999). Cerebellar cortical AMPA-kainate receptor blockade prevents performance of classically conditioned nictitating membrane responses.Journal of Neuroscience, 19(RC45) 1–6.
Attwell, P. J. E., Rahman, S., & Yeo, C. H. (2001). Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI.Journal of Neuroscience, 21, 5715–5722.
Backman, C., West, J. R., Mahoney, J. C., & Palmer, M. R. (1998). Electrophysiological characterization of cerebellar neurons from adult rats exposed to ethanol during development.Alcoholism: Clinical and Experimental Research, 22, 1137–1145.
Bao, S., Chen, L., Kim, J. J., & Thompson, R. F. (2002). Cerebellar cortical inhibition and classical eyeblink conditioning.Proceedings of the National Academy of Sciences, 99, 1592–1597.
Bayer, S. A., Altman, J., Russo, R. J., & Zhang, X. (1993). Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat.Neurotoxicology, 14, 83–144.
Berthier, N. E., & Moore, J. W. (1986). Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response.Experimental Brain Research, 63, 341–350.
Berthier, N. E., & Moore, J. W. (1990). Activity of deep cerebellar nuclear cells during classical conditioning of nictitating membrane extension in rabbits.Experimental Brain Research, 83, 44–54.
Bonthius, D. J., & West, J. R. (1990). Alcohol,-induced neuronal loss in developing rats: Increased brain damage with binge exposure.Alcoholism: Clinical and Experimental Research, 14, 107–118.
Bonthius, D. J., & West, J. R. (1991). Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt.Teratology, 44, 147–163.
Clark, G. A., McCormick, D. A., Lavond, D. G., & Thompson, R. F. (1984). Effects of lesions of cerebellar nuclei on conditioned behavioral and hippocampal neuronal responses.Brain Research, 291, 125–136.
Clark, R. E., Zhang, A. A., & Lavond, D. G. (1992). Reversible lesions of the cerebellar interpositus nucleus during acquisition and retention of a classically conditioned behavior.Behavioral Neuroscience, 106, 879–888.
Clark, R. E., Zhang, A. A., & Lavond, D. G. (1997). The importance of cerebellar cortex and facial nucleus in acquisition and retention of eyeblink/NM conditioning: Evidence for critical unilateral regulation of the conditioned response.Neurobiology of Learning and Memory, 67, 96–111.
Eccles, J. C., Llinas, R., & Sasaki, K. (1966). Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum.Experimental Brain Research, 1, 17–39.
Edwards, R. B., Manzana, E. J. P., & Chen, W.-J. A. (2002). Melatonin (an antioxidant) does not ameliorate alcohol-induced Purkinje cell loss in the developing cerebellum.Alcoholism: Clinical and Experimental Research, 26, 1003–1009.
Freeman, J. H., Barone, J., Stan, & Stanton, M. E. (1995). Disruption of cerebellar maturation by an antimitotic agent impairs the ontogeny of eyeblink conditioning in rats.Journal of Neuroscience, 15, 7301–7314.
Freeman, J. H., Carter, C. S., & Stanton, M. E. (1995). Early cerebellar lesions impair eyeblink conditioning in developing rats: Differential effects of unilateral lesions on postnatal day 10 or 20.Behavioral Neuroscience, 109, 893–902.
Freeman, J. H., & Nicholson, D. A. (1999). Neuronal activity in the cerebellar interpositus and lateral pontine nuclei during inhibitory classical conditioning of the eyeblink response.Brain Research, 833, 225–233.
Freeman, J. H., & Nicholson, D. A. (2000). Developmental changes in eye-blink conditioning and neuronal activity in the cerebellar interpositus nucleus.Journal of Neuroscience, 20, 813–819.
Freeman, J. H., Spencer, C. O., Skelton, R. W., & Stanton, M. E. (1993). Ontogeny of eyeblink conditioning in the rat: Effects of US intensity and interstimulus interval on delay conditioning.Psychobiology, 21, 233–242.
Garcia, K. S., & Mauk, M. D. (1998). Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses.Neuropharmacology, 37, 471–480.
Garcia, K. S., Steele, P. M., & Mauk, M. D. (1999). Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses.Journal of Neuroscience, 19, 10940–10947.
Goodlett, C. R., & Eilers, A. T. (1997). Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: A stereological study of temporal windows of vulnerability.Alcoholism: Clinical and Experimental Research, 21, 738–744.
Goodlett, C. R., & Horn, K. H. (2001). Mechanisms of alcohol-induced damage to the developing nervous system.Alcohol Research and Health, 25, 175–184.
Goodlett, C. R., & Johnson, T. B. (1999). Temporal windows of vulnerability to alcohol during the third trimester equivalent: Why “knowing when” matters. In J. H. Hannigan & L. P. Spear & N. E. Spear & C. R. Goodlett (Eds.),Alcohol and Alcoholism: Effects on Brain and Development (pp. 59–91). Hillsdale, NJ: Lawrence Erlbaum.
Goodlett, C. R., & Lundahl, K. R. (1996). Temporal determinants of neonatal alcohol-induced cerebellar damage and motor performance deficits.Pharmacology, Biochemistry and Behavior 55, 531–540.
Goodlett, C. R., Pearlman, A. D., & Lundahl, K. R. (1998). Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells.Neurotoxicology and Teratology, 20, 285–292.
Goodlett, C. R., Peterson, S. D., Lundahl, K. R., & Pearlman, A. D. (1997). Binge-like alcohol exposure of neonatal rats via intragastric intubation induces both Purkinje cell loss and cortical astrogliosis.Alcoholism: Clinical and Experimental Research, 21, 1010–1017.
Goodlett, C. R., Thomas, J. D., & West, J. R. (1991). Long-term deficits in cerebellar growth and rotarod performance of rats following “binge-like” alcohol exposure during the neonatal brain growth spurt.Neurotoxicology and Teratology, 13, 69–74.
Gould, T. J., & Steinmetz, J. E. (1996). Changes in rabbit cerebellar cortical and interpositus nucleus activity during acquisition, extinction, and backward classical eyelid conditioning.Neurobiology of Learning and Memory, 65, 17–34.
Green, J. T., Johnson, T. B., Goodlett, C. R., & Steinmetz, J. E. (2002). Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates.Learning & Memory, 9, 304–320.
Green, J. T., Rogers, R. F., Goodlett, C. R., & Steinmetz, J. E. (2000). Impairment in eyeblink classical conditioning in adult rats exposed to ethanol as neonates.Alcoholism: Clinical and Experimental Research, 24, 438–447.
Green, J. T., Tran, T., Steinmetz, J. E., & Goodlett, C. R. (2002). Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats.Brain Research, 956, 302–311.
Gruart, A., & Yeo, C. H. (1995). Cerebellar cortex and eyeblink conditioning: Bilateral regulation of conditioned responses.Experimental Brain Research, 104, 431–448.
Hamre, K. M., & West, J. R. (1993). The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles.Alcoholism: Clinical and Experimental Research, 17, 610–622.
Hardiman, M. J., & Yeo, C. H. (1992). The effect of kainic acid lesions of the cerebellar cortex on the conditioned nictitating membrane response in the rabbit.European Journal of Neuroscience, 4, 966–980.
Harvey, J. A., Welsh, J. P., Yeo, C. H., & Romano, A. G. (1993). Recoverable and nonrecoverable deficits in conditioned responses after cerebellar cortical lesions.Journal of Neuroscience, 13, 1624–1635.
Heaton, M. B., Mitchell, J. J., & Paiva, M. (2000). Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy.Alcoholism: Clinical and Experimental Research, 24, 512–518.
Hesslow, G., & Ivarsson, M. (1994). Suppression of cerebellar Purkinje cells during conditioned responses in ferrets.NeuroReport, 5, 649–652.
Ito, M. (1984).The Cerebellum and Motor Control. New York: Raven Press.
Jones, K. L., & Smith, D. W. (1973). Recognition of the fetal alcohol syndrome in early infancy.Lancet, 2, 999–1001.
Katz, D. B., & Steinmetz, J. E. (1997). Single-unit evidence for eye-blink conditioning in cerebellar cortex is altered, but not eliminated, by interpositus nucleus lesions.Learning and Memory, 3, 88–104.
Klintsova, A. Y., Cowell, R. M., Swain, R. A., Napper, R. M. A., Goodlett, C. R., & Greenough, W. T. (1998). Therapeutic effects of complex motor training on motor performance deficits induced by neonatal bingelike alcohol exposure in rats: I. Behavioral results.Brain Research, 800, 48–61.
Klinstova, A. Y., Matthews, J. T., Goodlett, C. R., Napper, R. M. A., & Greenough, W. T. (1997). Therapeutic motor training increases parallel fiber synapse number per Purkinje neuron in cerebellar cortex of rats given postnatal binge alcohol exposure: Preliminary report.Alcoholism: Clinical and Experimental Research, 21, 1257–1263.
Klintsova, A. Y., Scamra, C., Hoffman, M., Napper, R. M. A., Goodlett, C. R., & Greenough, W. T. (2002). Therapeutic effects of complex motor training on motor performance deficits induced by neonatal bingelike alcohol exposure in rats: II. A quantitative stereological study of synaptic plasticity in female rat cerebellum.Brain Research, 937, 83–93.
Krupa, D. J., Thompson, J. K., & Thompson, R. F. (1993). Localization of a memory trace in the mammalian brain.Science, 260, 989–991.
Lavond, D. G., Hembree, T. L., & Thompson, R. F. (1985). Effect of kainic acid lesions of the cerebellar interpositus nucleus on eyelid conditioning in the rabbit.Brain Research, 326, 179–182.
Lavond, D. G., Lincoln, J. S., McCormick, D. A., & Thompson, R. F. (1984). Effects of bilateral lesions of the dentate and interpositus cerebellar nuclei on conditioning of heart-rate and nictitating membrane/eyelid responses in the rabbit.Brain Research, 305, 323–330.
Lavond, D. G., & Steinmetz, J. E. (1989). Acquisition of classical conditioning without cerebellar cortex.Behavioural Brain Research, 33, 113–164.
Lavond, D. G., Steinmetz, J. E., Yokaitis, M. H., & Thompson, R. F. (1987). Reacquisition of classical conditioning after removal of cerebellar cortex.Experimental Brain Research, 67, 569–593.
Lemoine, P., Harouseau, H., Borteryu, J. T., & Menuet, J. C. (1968). Les enfants des parents alcooliques: Anomalies observees apropos de 127 cas.Ouest Medical, 21, 476–482.
Lincoln, J. S., McCormick, D. A., & Thompson, R. F. (1982). Ispilateral cerebellar lesions prevent learning of the classically conditioned nictitating membrane/eyelid response.Brain Research, 242, 190–193.
Maier, S. E., Miller, J. A., Blackwell, J. M., & West, J. R. (1999). Fetal alcohol exposure and temporal vulnerability: Regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development.Alcoholism: Clinical and Experimental Research, 23, 726–734.
Marcussen, B. L., Goodlett, C. R., Mahoney, J. C., & West, J. R. (1994). Developing rat Purkinje cells are more vulnerable to alcohol-induced depletion during differentiation than during neurogenesis.Alcohol, 11, 147–156.
Marr, D. (1969). A theory of cerebellar cortex.Journal of Physiology, 202, 437–470.
McCormick, D. A., Clark, G. A., Lavond, D. G., & Thompson, R. F. (1982). Initial localization of the memory trace for a basic form of learning.Proceedings of the National Academy of Sciences, 79, 2731–2735.
McCormick, D. A., Lavond, D. G., Clark, G. A., Kettner, R. R., Rising, C. E., & Thompson, R. F. (1981). The engram found? Role of the cerebellum in classical conditioning of nictitating membrane and eyelid responses.Bulletin of the Psychonomic Society, 18, 103–105.
McCormick, D. A., Steinmetz, J. E., & Thompson, R. F. (1985). Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response.Brain Research, 359, 120–130.
McCormick, D. A., & Thompson, R. F. (1984). Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response.Journal of Neuroscience, 4, 2811–2822.
Medina, J. F., Garcia, K. S., Nores, W. L., Taylor, N. M., & Mauk, M. D. (2000). Timing mechanisms in the cerebellum: Testing predictions of a large-scale computer simulation.Journal of Neuroscience, 20, 5516–5525.
Meyer, L. S., Kotch, L. E., & Riley, E. P. (1990). Neonatal ethanol exposure: Functional alterations associated with cerebellar growth retardation.Neurotoxicology and Teratology, 12, 15–22.
Miki, T., Harris, S., Wilce, P., Takeuchi, Y., & Bedi, K. S. (1999). The effect of the timing of ethanol exposure during early postnatal life on total number of Purkinje cells in rat cerebellum.Journal of Anatomy, 194, 423–431.
Napper, R. M. A., & West, J. R. (1995a). Permanent neuronal cell loss in the cerebellum of rats exposed to continuous low blood alcohol levels during the brain growth spurt: A stereological investigation.Journal of Comparative Neurology, 362, 283–292.
Napper, R. M. A., & West, J. R. (1995b). Permanent neuronal cell loss in the inferior olive of adult rats exposed to alcohol during the brain growth spurt: A stereological investigation.Alcoholism: Clinical and Experimental Research, 19, 1321–1326.
Ohyama, T., & Mauk, M. D. (2001). Latent acquisition of timed responses in cerebellar cortex.Journal of Neuroscience, 21, 682–690.
Paczkowski, C., Ivkovich, D., & Stanton, M. E. (1999). Ontogeny of eyeblink conditioning using a visual conditional stimulus.Developmental Psychobiology, 35, 253–263.
Perrett, S. P., & Mauk, M. D. (1995). Extinction of conditioned eyelid responses requires the anterior lobe of cerebellar cortex.Journal of Neuroscience, 15, 2074–2080.
Perrett, S. P., Ruiz, B. P., & Mauk, M. D. (1993). Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses.Journal of Neuroscience, 13, 1708–1718.
Pierce, D. R., Serbus, D. C., & Light, K. E. (1993). Intragastric intubation of alcohol during postnatal development of rats results in selective cell loss in the cerebellum.Alcoholism: Clinical and Experimental Research, 17, 1275–1280.
Rogers, R. F., Britton, G. B., & Steinmetz, J. E. (2001). Learning-related interpositus activity is conserved across species as studies during eyeblink conditioning in the ratBrain Research, 905, 171–177.
Rossebo, O. E., Green, J. T., & Steinmetz, J. E. (2002). Neonatal exposure to ethanol causes dose-dependent deficits in eyeblink conditioning in the adult rat.Society for Neuroscience Abstracts, 28.
Ryabinin, A. E., Cole, M., Bloom, F. E. & Wilson, M. C. (1995). Exposure of neonatal rats to alcohol by vapor inhalation demonstrates specificity of microcephaly and Purkinje cell loss but not astrogliosis.Alcoholism: Clinical and Experimental Research, 19, 784–791.
Skelton, R. W. (1988). Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats.Behavioral Neuroscience, 102, 586–590.
Stanton, M. E., Fox, G. D., & Carter, C. S. (1998). Ontogeny of the conditioned eyeblink response in rats Acquisition or expression?Neuropharmacology, 37, 623–632.
Stanton, M. E., Freeman, J. H., & Skelton, R. W. (1992). Eyeblink conditioning in the developing rat.Behavioral Neuroscience, 106, 657–665.
Stanton, M. E., & Goodlett, C. R. (1998). Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats.Alcoholims: Clinical and Experimental Research, 22, 270–275.
Steinmetz, J. E., Lavond, D. G., Ivkovich, D, Logan, C. G., & Thompson, R. F. (1992) Disruption of classical eyelid conditioning after cerebellar lesions: Damage to a memory trace system or a simple performance deficit?Journal of Neuroscience, 12, 4403–4426.
Steinmetz, J. E., Logan, C. G., Rosen, D. J., Thompson, J. K., Lavond, D. G., & Thompson, R. F. (1987). Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning.Proceedings of the National Academy of Sciences, 84, 3531–3535.
Stratton, K., Howe, C., & Battaglia, F. (1996).Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC: National Academy Press.
Thach, W. T. (1967). Somatosensory receptive fields of single units in cat cerebellar cortex.Journal of Neurophysiology, 30, 675–696.
Thomas, J. D., Goodlett, C. R., & West, J. R. (1998). Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates, with motor performance.Developmental Brain Research, 105, 159–166.
Thomas, J. D., Wasserman, E. A., West, J. R., & Goodlett, C. R. (1996). Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: Importance of developmental timing and number of episodes.Developmental Psychobiology, 29(5), 433–452.
Woodruff-Pak, D. S., Lavond, D. G., Logan, C. G., Steinmetz, J. E., & Thompson, R. F. (1993). Cerebellar cortical lesions and reacquisition in classical conditioning of the nictitating membrane response in rabbits.Brain Research 608, 67–77.
Yeo, C. H., & Hardiman, M. J. (1992). Cerebellar cortex and eyeblink conditioning: A reexamination.Experimental Brain Research, 88, 623–638.
Yeo, C. H., Hardiman, M. J., & Glickstein, M. (1985). Classical conditioning of the nictitating membrane response, of the rabbit II. Lesions of the cerebellar cortex.Experimental Brain Research, 60, 99–113.
Yeo, C. H., Hardiman, M. J., & Glickstein, M. (1986). Classical conditioning of the nictitating membrane response of the rabbit IV. Lesions of the inferior olive.Experimental Brain Research, 63, 81–92.
Young, B. W., Green, J. T. & Steinmetz, J. E. (2002). Physiological effects of neonatal alcohol-induced Purkinje cell loss in adult rats.Society for Neuroscience Abstracts, 28.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, J.T. Using eyeblink classical conditioning as a test of the functional consequences of exposure of the developing cerebellum to alcohol. Integrative Physiological & Behavioral Science 38, 45–64 (2002). https://doi.org/10.1007/BF02734260
Issue Date:
DOI: https://doi.org/10.1007/BF02734260