Abstract
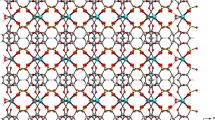
A hydrothermal reaction of a mixture of Y(NO3)3, 1,2-benzenedicarboxylic acid (1,2-BDC) and NaOH gives rise to a new yttrium phthalate coordination polymer, [Y4H2O2C8H4O4)6]∞,I. The Y ions inI are present in four different coordination environments with respect to the oxygen atoms (CN6 = octahedral, CN7 = pentagonal bipyramid, CN8 = dodecahedron and CN9 = capped square anti-prism). The oxygen atoms of the 1,2-BDC are fully deprotonated, and show variations in their connectivity with Y atoms. The Y atoms themselves are connected through their vertices forming infinite Y-O-Y one-dimensional chains. The Y-O-Ychains are cross-linked by the 1,2-BDC anions forming a corrugated layer structure. The layers are supported by favourableπ…π interactions between the benzene rings of the 1,2-BDC anions. The variations in the coordination environment of the Y atoms and the presence of Y-O-Y interactions along with the favourableπ…π interactions between the benzene rings from different layers are noteworthy structural features. Crystal data: triclinic, space group =P−1 (no. 2),a = 12.6669 (2),b = 13.8538 (2),c = 16.0289 Å,α = 75.20 (1),β = 69.012 (1),γ= 65.529 (1)°,V = 2371.28 (7) Å3,D calc = 1.922 g cm−1, μ(MoKα) = 4.943 mm−1. A total of 9745 reflections collected and merged to give 6566 unique reflections (R int = 0.0292) of which 5252 withI>2σ(I) were considered to be observed. FinalR 2 = 0.0339,wR 2 = 0.0724 andS = 1.036 were obtained for 704 parameters.
Similar content being viewed by others
References
Eddaoudi M, Moler D B, Li H, Chen B, Reineke T M, O’Keeffe M and Yaghi O M 2001Acc. Chem. Res. 34 319
Cao R, Sun D, Liang Y, Hong M, Tatsumi K and Shi Q 2002Inorg. Chem. 41 2087
Pan L, Zheng N, Wu Y, Han S, Yang R, Huang X and Li J 2001Inorg. Chem. 40 828
Long D L, Blake A J, Champness N R and Schroder M 2000Chem. Commun. 1369
Liang Y-C, Cao R, Su W-P, Hong M-C and Zhang W-J 2000Angew. Chem. Int. Ed. 39 3304
Reineke T M, Eddaoudi M, Fehr M, Kelley D and Yaghi O M 1999J. Am. Chem. Soc. 121 1651
Goodgame D M L, Menzer S, Ross A T and Williams D J 1994Chem. Commun. 2605
Decurtins S, Gross M, Schmalle H W and Ferlay S 1998Inorg. Chem. 37 2443
Kiritsis V, Michaelides A, Skoulika S, Golhen S and Quahab L 1998Inorg. Chem. 37 3407
Serpaggi F and Ferey G 1998J. Mater. Chem. 8 2737
Shibasaki M, Yamada K-I and Yoshikawa N 1999Lewis acids in organic synthesis (ed.) H Yamamoto (New York: Wiley-VCH) vol. 2
Imamoto T 1994Lanthanide in organic synthesis (New York: Academic Press)
Sheldrick G M 1997 SADABS user guide, Siemens area detector absorption correction program. University of Gottingen, Gottingen
Sheldrick GM 2001 SHELXLTL-PLUS, ver. 6.12 Bruker AXS Inc, Madison, Wisconson
Wang Y, Jin L, Wang K, Zhang L, Zheng X and Lu Z 2002New J. Chem. 26 1590
Hunter C A, Singh J and Thronton J M 1991J. Mol. Biol. 218 837
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor C N R Rao on his 70th birthday
Rights and permissions
About this article
Cite this article
Thirumurugan, A., Natarajan, S. A two-dimensional yttrium phthalate coordination polymer, [Y4(H2O)2(C8H4O4)6]∞, exhibiting different coordination geometries. J Chem Sci 115, 573–586 (2003). https://doi.org/10.1007/BF02708249
Issue Date:
DOI: https://doi.org/10.1007/BF02708249