Abstract
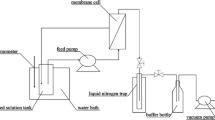
Polyetherimide/γ-alumina composite membrane has been prepared by dipping method for further reactor application. Separation factors and permeances for a quaternary acetic acid-ethanol-ethyl acetate-water equilibrium feed mixture have been measured on the composite membrane in the range of temperature from 303 K up to 343 K and space time from 27 sec to 27,000 sec at a permeate-side pressure of 2.67×10-3 bar. Fluxes for the all four components increase as temperature inicreases due to the increase of free volume within polymer chains. Apparent activation energies have been measured for the four components. The flux change for acetic acid is the most sensitive on the temperature change. The composite membrane shows a good dehydration capability with reasonable separation characteristics, as well as thermal and mechanical stability in desired conditions.
Similar content being viewed by others
References
Chang, J., Yoo, J., Ahn, S., Lee, K. and Ko, S.,“Simulation of Pervaporation Process for Ethanol Dehydration by Using Pilot Test Results”Korean J. Chem. Eng.,15, 28 (1998).
Chen, G. and Yuan, Q.,“Methanol Synthesis from CO2 Using a Silicon Rubber/Ceramic Composite Membrane Reactor,”Sep. and Puri. Technol,34, 227 (2004).
Kim, S. G., Jegal, J. G. and Lee, K. H.,“Study on the Pervaporation Characteristics of Water-Alcohol Mixtures through Aromatic Polyetherimide Membranes: II. The Pervaporation of Water Isopropanol Mixtures by the Density Change of Skin Layer,”J. Korean Ind. & Eng. Chem.,8, 853 (1997).
Lim, S. Y., Park, B., Hung, F., Sahimi, M. and Tsotsis, T. T.,“Design Issues of Pervaporation Membrane Reactors for Esterifications,”Chem. Eng. Sci.,57, 4933 (2002).
Mulder, M. H. V,“Basic Prinsiples of Membrane Technolpgy,” Kluwer Academic Publishers, Dordrecht, The Netherlands (1996).
Muramatsu, M., Okura, M., Kuboyama, K., Ougizawa, T., Yamamoto, T., Nishihara, Y., Saito, Y., Ito, K., Hirata, K. and Kobayashi, Y., “Oxygen Permeability and Free Volume Hole Size in Ethylene-Vinyl Alcohol Copolymer Film: Temperature and Humidity Dependence,”Radiation Phys. and Chem.,68, 561 (2003).
Okamoto, K., Tanihara, N., Watanabe, H., Tanaka, K., Kita, H., Nakamura, A., Kusuki, Y. and Nakagawa, K.,“Vapor Permeation and Pervaporation Separation of Water-Ethanol Mixtures through Polyimide Membranes,”J. Membrane Sci.,68, 53 (1992).
Park, B., Ravi-Kumar, V.S. and Tsotsis, T. T.,“Models and Simulation of Liquid-Phase Membrane Reactors,”Ind. Eng. Chem. Res.,37, 1276 (1998).
Park, B. and Tsotsis, T. T.,“Models and Experiments with Pervaporation Membrane Reactors Integrated with an Adsorbent System,”Chem. Eng. and Proc,43, 1171 (2004).
Park, C. and Geng, C. Q.,“Mathematical Modeling of Fed-Batch Butanol Fermentation with Simultaneous Pervaporation,”Korean J. Chem. Eng.,13, 612 (1996).
Prausnitz, J. M., Lixhtenthaler, R. N. and Azevedo, E. G.,“Molecular Thermodynamics of Fluid-Phase Equilibria,” Prentice Hall, Inc., Englewood Cliffs, New Jersey (1986).
Qariouh, H., Schue, R. and Bailly, C.,“Sorption, Diffusion and Pervaporation of Water/Ethanol Mixtures in Polyetherimide Membranes,”Polymr. Int.,48, 171 (1999).
Song, K. M., Hong, Y. K., Yu, J. and Hong, W. H.,“Influence of Temperature Drop by Phase Transition on Pervaporation Processes in Vapor Phase Feed,”Korean J. Chem. Eng.,19, 290 (2002).
Spillman, R. W.,“Economics of Gas Separation Membrane,”Chem. Eng. Prog.,January, 41 (1989).
Waldburger, R. M. and Widmer, F.,“Membrane Reactors in Chemical Production Processes and the Application to the Pervaporation-Assisted Esterification,”Chem. Eng. Technol.,19, 117 (1996).
Wang, X., Shen, Z., Zhang, F. and Zhang, Y.,“Preferential Separation of Ethanol from Aqueous Solution through Hydrophilic Polymer Membranes,”Appl. Polymr. Sci.,73, 1145 (1999).
Won, J. M., Bae, S. Y., Ha, B. H., Kim, H. T. and Kumazawa, H.,“Plasticization of Chitosan Membrane for Pervaporation of Aqueous Ethanol Solution,”Korean J. Chem. Eng.,13, 324 (1996).
Yeom, C., Dickson, J. M. and Brook, M. A.,“A Characterization of PDMS Pervaporation Membranes for the Removal of Trace Organic from Water,”Korean J. Chem. Eng.,13, 482 (1996).
Yoshida, W. and Cohen, Y., “Ceramic-Supported Polymer Membranes for Pervaporation of Binary Organic/Organic Mixtures,”J. Membrane Sci. 213, 145 (2003).
Zhu, Y., Minet, R. G. and Tsotsis, T. T.,“A Continuous Pervaporation Membrane Reactor for the Study of Esterifications Reactions Using a Composite Polymeric/Ceramic Membrane,”Chem. Eng. Sci.,51, 4103 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, BG. Pervaporation characteristics of polyetherimide/γ-alumina composite membrane for a quaternary equilibrium mixture of acetic acid-ethanol-ethyl acetate-water. Korean J. Chem. Eng. 21, 882–889 (2004). https://doi.org/10.1007/BF02705534
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705534