Summary
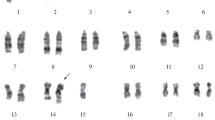
A variant nontransformed clone, I21, was selected from the spontaneously transformed mouse fibroblast line, IT22. Selection was done by plating IT22 in methylcellulose and picking single cells after 2 d. Cultures derived from these single cells were selected again and one clone, I21, derived from the second round of selection was characterized extensively. I21 and IT22 have the same plating efficiency (PE) on plastic, but in agarose they differ by 1000-fold. In comparison to IT22, I21 has a normal morphological appearance, a lower saturation density, a higher viability in stationary phase, an increased doubling time, an increased chromosome content, and is unable to form tumors in nude mice. I21 has remained remarkably stable in culture and has not reverted to the transformed phenotype for at least 300 generations in culture. Over 100 clones of I21, expanded to 106 cells, failed to show an increased PE in agarose. Even expansion of the rare colonies of I21 that grow in agarose failed to produce clones similar to IT22.
Similar content being viewed by others
References
Gey, G. O. Cytological and cultural observations on transplantable rat sarcomata produced by inoculation of altered normal cells maintained in continuous culture. Cancer Res. 1: 737; 1941.
Earle, W. R.; Nettleship, A. Production of malignancy in vitro. V. Results of injections of cultures into mice. J. Natl. Cancer Inst. 4: 213–227; 1943.
Sanford, K. Malignant transformation of cells in vitro. Int. Rev. Cytol. 18: 249–311; 1965.
Ponten, J. Spontaneous and virus-induced transformation in culture. In: Gard, S.; Hallauer, C.; Meyer, K. F. eds. Virology monographs. Wien, New York: Springer-Verlag; 1971: 1–45.
Pollack, R.; Wolman, S.; Vogel, A. Reversion of virus-transformed cell lines: hyperploidy accompanies retention of viral genes. Nature 228: 967–970; 1970.
Marshall, C. J.; Dave, H. Suppression of the transformed phenotype in somatic cell hybrids. J. Cell Sci. 33: 171–190; 1978.
MacPherson, I.; Montagnier, L. Agar suspension culture for the selective assay of cells transformed by polyoma virus. Virology 23: 291–294; 1964.
Shin, S.; Freedman, V. H.; Risser, R.; Pollack, R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc. Natl. Acad. Sci. USA 72: 4435–4439; 1975.
Vogel, A.; Pollack, R. Methods of obtaining revertants of transformed cells. In: Prescott, D. M., ed. Methods in cell biology. Vol. 8. New York, London: Academic Press; 1974: 75–92.
Todaro, G. J.; Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established cell lines. J. Cell Biol. 17: 299–313; 1963.
Croce, C. M.; Knowles, B. B.; Koprowski, H. Preferential retention of the human chromosome C-7 in human-(thymidine kinase deficient) mouse hybrid cells. Exp. Cell Res. 82: 457–461; 1973.
Stanners, C. P.; Elicieri, G. L.; Green, H. Two types of ribosome in mouse-hamster hybrids. Nature 230: 52–53; 1971.
Levine, E. M.; Becker, B. G. Biochemical methods for detectingMycoplasma contamination. In: McGarrity, G. J.; Murphy, D. G.; Nichols, W. W., eds.Mycoplasma infection of cell cultures, New York: Plenum Press; 1978: 87–104.
Vogel, A.; Pollack, R. Isolation and characterization of revertant cell lines. IV. Direct selection of serum-revertant sublines of SV40-transformed 3T3 mouse cells. J. Cell Physiol. 82: 189–198; 1973.
Hitosumachi, S.; Rabinowitz, Z.; Sachs, L. Chromosomal control of reversion in transformed cells. Nature 225: 511–514; 1971.
Rabinowitz, Z.; Sachs, L. Control of reversion of properties in transformed cells. Nature 225: 136–139; 1970.
Pollard, J. W.; Stanners, C. P. Characterization of cell lines showing growth control isolated from both the wild-type and a leucyl-tRNA synthetase mutant of Chinese hamster ovary cells. J. Cell Physiol. 98: 571–586; 1979.
MacPherson, I. Reversion in hamster cells transformed by Rous sarcoma virus. Science 148: 1731–1733; 1965.
Stephenson, R. R.; Scolnik, E. M.; Aaronson, S. A. Genetic stability of the sarcoma viruses in murine and avian sarcoma virus-transformed non-producer cells. Int. J. Cancer 9: 577–583; 1972.
Ozanne, B.; Vogel, A. Selection of revertants of Kirsten sarcoma virus transformed nonproducer BALB/3T3 cells. J. Virol. 14: 239–248; 1974.
Sager, R.; Kovac, P. E. Genetic analysis of tumorigenesis. I. Expression of tumor-forming ability in hamster hybrid cell lines. Somat. Cell Genet. 4: 375–392; 1978.
Graham, F. L.; Bacchetti, S.; McKinnon, R.; Stanners, C.; Cordell, B.; Goodman, H. Transformation of mammalian cells with DNA using the calcium technique. In: Baserga, R.; Croce, C.; Rovera, G. eds. Introduction of macromolecules into viable mammalian cells. The Wistar symposium series. Vol. 1. New York: Alan R. Liss, Inc.; 1980: 3–25.
Cooper, G. M. Cellular transforming genes. Science 218: 801–806; 1982.
Murray, M. J.; Shilo, B.-Z.; Shih, C.; Cowing, D.; Hsu, H. W.; Weinberg, R. A. Three different human tumor cell lines contain different oncogenes. Cell 25: 355–361; 1981.
Shimizu, K.; Goldfarb, M.; Suard, Y.; Perucho, M.; Li, Y.; Kamata, T.; Feramisco, J.; Stavnezer, E.; Fogh, J.; Wigler, M. H. Three human transforming genes are related to the viral ras oncogenes. Proc. Natl. Acad. Sci. USA 80: 2112–2116; 1983.
Author information
Authors and Affiliations
Additional information
The research was supported by the Medical Research Council and the National Cancer Institute of Canada. R. Godbout was supported by a 1967 Science Scholarship and by an MRC Studentship. B. L. Gallie is a Research Associate of the Ontario Cancer Treatment and Research Foundation.
Rights and permissions
About this article
Cite this article
Godbout, R., Gallie, B.L. & Phillips, R.A. Characterization of a stable, anchorage-dependent clone obtained from a spontaneously transformed mouse cell line. In Vitro 20, 479–485 (1984). https://doi.org/10.1007/BF02619621
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02619621