Abstract
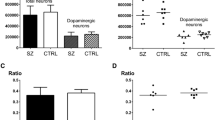
The phenotypic effect of theweaver mutation in the ventral midbrain of homozygous mutants is associated with the progressive loss of dopaminergic neurons. To discover whether the number of mesencephalic dopaminergic cells is altered in weaver heterozygotes (wv/+), we studied mice between 20 and 365 days of age. We counted tyrosine hydroxylase (TH)-immunopositive cells in the substantia nigra (SN), retrorubral nucleus (RRN), and ventral tegmental area (VTA), and measured cross-sectional areas of neuronal somata in the SN ofwv/+ and age-matched wild-type controls (+/+). The number of TH-positive cells in thewv+ ventral midbrain was on average 13% lower than normal. Cell loss was detected selectively in the SN (12%) and VTA (23%). The areas of somatic profiles in thewv/+ nigral neurons were on average reduced by 9.8%. The neuronal losses in the SN and VTA correlated with a 13.8% reduction in dopamine level in the ventral striatum inwv/+ mice at 14–16 months of age. Our findings imply that a single dose of theweaver gene in the mouse is associated with cellular damage leading to a chronic deficiency in the mesostriatal dopaminergic system.
Similar content being viewed by others
References
Alfert M, Bern HA, Kahn RD (1955) Hormonal influence on nuclear synthesis. Acta Anat 23:185
Bayer SA, Triarhou LC, Thomas JD, Ghetti B (1994) Correlated quantitative studies of the neostriatum, nucleus accumbens, substantia nigra, and ventral tegmental area in normal and weaver mutant mice. J Neurosci 14:6901–6910
Bayer SA, Wills KV, Triarhou LC, Verina T, Thomas JD, Ghetti B (1995) Selective vulnerability of late-generated dopaminergic neurons of the substantia nigra in weaver mutant mice. Proc Natl Acad Sci USA 92:9137–9140
Björklund A, Lindvall O (1984) Dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, vol 2. Elsevier, Amsterdam, pp 55–122
Blatt GJ, Eisenman LM (1985) A qualitative and quantitative light microscopic study of the inferior olivary complex of normal, reeler, and weaver mutant mice. J Comp Neurol 232:117–128
Bostwick JR, Le WD (1991) A tyrosine hydroxylase assay in microwells using coupled nonenzymatic decarboxylation of DOPA. Anal Biochem 192:125–130
Gaspar P, Ben Jelloun N, Febvret A (1994) Sparing of the dopaminergic neurons containing calbindin-D28K and of the dopaminergic mesocortical projections in weaver mutant mice. Neuroscience 61:293–305
German DC, Manaye KF, Sonsalla PK, Brooks BA (1992) Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced Parkinsonism: sparing of calbindin-D28K-containing cells. Ann NY Acad Sci 648:42–62
Ghetti B, Triarhou LC (1990) Profile of mesencephalic dopamine neuron loss in weaver mutant mice during life-span. Soc Neurosci Abstr 16:1138
Ghetti B, Triarhou LC (1992a) Nigrostriatal aberrations induced by weaver gene are present at birth. Soc Neurosci Abstr 18:156
Ghetti B., Triarhou LC (1992b) Combined degeneration of cerebellar granule cells and of midbrain dopamine neurons in the weaver mutant mouse. In: Hefti F, Weiner WJ (eds) Progress in Parkinson’s disease research. Futura Publishing, Mount Kisko, NY, pp 375–388
Ghetti B, Triarhou LC (1992c) Degeneration of mesencephalic dopamine neurons in weaver mutant mice. Neurochem Int 20:305S-307S
Gupta M, Felten DL, Ghetti B (1987) Selective loss of monoaminergic neurons in weaver mutant mice: an immunocytochemical study. Brain Res 402:379–382
Hartman HA, Kirsch GE, Drewe JA, Taglialatela M, Joho RH, Brown AM (1991) Exchange of conduction pathways between two related K+ channels. Science 251:942–944
Heginbotham L, Abramson T, MacKinnon R (1992) A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science 258:1152–1155
Horowski R, Wachtel H, Turski L, Löschmann P-A (1994) Glutamate excitotoxicity as a possible pathogenetic mechanism of chronic neurodegeneration. In: Calne DB (ed) Neurodegenerative diseases. WB Saunders, Philadelphia, pp 163–175
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lundberg C, Wictorin K, Björklund A (1994) Retrograde degenerative changes in the substantia nigra pars compacta following an excitotoxic lesion of the striatum. Brain Res 644:205–212
Maragos WF, Greenamyre JT, Penney JB, Young AB (1987) Glutamate dysfunction in Alzheimer’s disease: an hypothesis. Trends Neurosci 10:65–68
Meldrum BS, Garthwaite J (1990) Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci 11:379–387
Palkovits M, Fischer J (1968) Karyometric investigation. Akademiai Kiado Budapest
Patil N, Cox DR, Bhat D, Faham M, Myers RM, Peterson A (1995) A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nature Genet 11:126–129
Rakic P, Sidman RL (1973a) Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex in weaver mutant mice. J Comp Neurol 152:103–132
Rakic P, Sidman RL (1973b) Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J Comp Neurol 152:133–162
Rezai Z, Yoon CH (1972) Abnormal rate of granule cell migration in the cerebellum of weaver mutant mice. Dev Biol 29:17
Richter JA, Stotz EH, Ghetti B, Simon JR (1992) Comparison of alterations in tyrosine hydroxylase, dopamine level and dopamine uptake in the striatum of the weaver mutant mouse. Neurochem Res 17:437–441
Roffler-Tarlov S (1992) Genetic causes of neuronal cell death. Ann NY Acad Sci 648:105–118
Roffler-Tarlov S, Graybiel AM (1984) Weaver mutation has differential effects on the dopamine-containing innervation of the limbic and non-limbic striatum. Nature 307:62–66
Roffler-Tarlov S, Graybiel AM (1986) Expression of the weaver gene in dopamine-containing neural systems is dose-dependent and affects both striatal and nonstriatal regions. J Neurosci 6:3319–3330
Roffler-Tarlov S, Graybiel AM (1987) The postnatal development of the dopamine-containing innervation of dorsal and ventral striatum: effects of the weaver gene. J Neurosci 7:2364–2372
Roffler-Tarlov S, Graybiel AM (1991) Genetic effects of dopamine uptake sites in the striatum. Soc Neurosci Abstr 17:1289
Roffler-Tarlov S, Pugatch D, Graybiel AM (1990) Patterns of cell and fiber vulnerability in the mesostriatal system of the mutant mouse weaver. II. High affinity uptake sites for dopamine. J Neurosci 10:734–740
Roffler-Tarlov S, Martin B, Graybiel AM, Kauer JS (1996) Cell death in the midbrain of the murine mutation weaver. J Neurosci 16:1819–1826
SAS Institute (1989) SAS/STAT user’s guide, version 6. SAS Institute, Cary, NC
Scharfman HE, Schwartzkroin PA (1989) Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science 246:257–260
Schmidt MJ, Sawer BD, Perry KW, Fuller RW, Foreman MM, Ghetti B (1982) Dopamine deficiency in the weaver mutant mouse. J Neurosci 2:376–380
Sidman RL (1968) Development of interneuronal connections in brains of mutant mice. In: Carlson FD (ed) Physiological and biochemical aspects of nervous integration. Prentice-Hall, Englewood Cliffs, pp 163–193
Sidman RL (1982) Mutations affecting the central nervous system in the mouse. In: Schmidt FO, Bird SJ, Bloom FE (eds) Molecular genetic neuroscience. Raven Press, New York, pp 389–400
Simon JR, Ghetti B (1992) Topographic distribution of dopamine uptake, choline uptake, choline acetyltransferase, and GABA uptake in the striata of weaver mutant mice. Neurochem Res 17:431–436
Simon JR, Richter JA, Ghetti B (1994) Age-dependent alterations in dopamine content, tyrosine hydroxylase activity, and dopamine uptake in the striatum of the weaver mutant mouse. J Neurochem 62:543–548
Slesinger PA, Patil N, Liao YJ, Jan LY, Cox DR (1996) Functional effects of the weaver mutation on the G protein-gated inwardly rectifying K+ channels. Neuron 16:321–331
Smeyne RJ, Goldowitz D (1990) Purkinje cell loss is due to a direct action of the weaver gene in Purkinje cells: evidence from chimeric mice. Dev Brain Res 52:211–218
Smith MW, Cooper TR, Joh TH, Smith DE (1990) Cell loss and class distribution in the substantia nigra of the neurological mutant, weaver. Brain Res 510:242–250
Sotelo C (1980) Mutant mice and the formation of the cerebellar circuitry. Trends Neurosci 3:33–36
Sternberger LA, Hardy PH Jr, Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Stotz EH, Palacios JM, Landwehrmeyer B, Norton J, Ghetti B, Simon JR, Triarhou LC (1994) Alterations in dopamine and serotonin uptake systems in the striatum of the weaver mutant mouse. J Neural Transm Gen Sect 97:51–64
Tong Y, Wei J, Wang Y, Zhang S, Strong J, Dlouhy SR, Hodes ME, Ghetti B, Yu L (1996) The weaver mutation changes the ion selectivity of the affected inwardly rectifying potassium channel GIRK2. FEBS Lett 390:63–68
Triarhou LC (1987) Definition of the mesostriatal dopamine deficit in the weaver mutant mouse and reconstruction of the damaged pathway by means of neural transplantation. Doctoral dissertation Indiana University. Ann Arbor (University Microfilm International, no. 8727536)
Triarhou LC (1992) Weaver gene expression in central nervous system. Methods Neurosci 9:209–227
Triarhou LC, Ghetti B (1989) The dendritic dopamine projection of the substantia nigra: phenotypic denominator of weaver gene action in hetero- and homozygosity. Brain Res 501:373–381
Triarhou LC, Ghetti B (1991) Further characterization of the dopaminergic dendrite deficit in substantia nigra pars reticulata of heterozygous and homozygous weaver mutant mice: Golgi, MAP2 and synaptic connectivity studies. Soc Neurosci Abstr 17:159
Triarhou LC, Low WC, Ghetti B (1986) Transplantation of ventral mesencephalic anlagen to hosts with genetic nigrostriatal dopamine deficiency. Proc Natl Acad Sci USA 83:8789–8793
Triarhou LC, Norton J, Ghetti B (1988) Mesencephalic dopamine cell deficit involves areas A8, A9 and A10 in weaver mutant mice. Exp Brain Res 70:256–265
Trump BF, Berezesky IK (1992) The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr Opin Cell Biol 4:227–232
Verina T, Tang X, Fitzpatrick L, Norton J, Vogelweid C, Ghetti B (1995) Degeneration of Sertoli and spermatogenic cells in homozygous and heterozygous weaver mice. J Neurogenet 9:251–265
Verney C, Febvret-Muzerelle A, Gaspar P (1995) Early postnatal changes of the dopaminergic mesencephalic neurons in the weaver mutant mouse. Dev Brain Res 89:115–119
Williams RW, Herrup C (1988) The control of neuron number. Annu Rev Neurosci 11:423–453
Winer BJ, Brown DR, Michels KM (1971), Statistical principles in experimental design, 3rd ed. McGraw-Hill, New York
Yamada T, McGeer PL, Baimbridge KG, McGeer EG (1990) Relative sparing in Parkinson’s disease of substantia nigra dopamine neurons containing calbindin-D28K. Brain Res 526:303–307
Yool AJ, Schwarz TL (1991) Alteration of ionic selectivity of K+channel by mutation of the H5 region. Nature 349:700–704
Zanjani HS, Mariani J, Delhaye-Bouchaud N, Herrup K (1991) Neuronal loss in heterozygous staggerer mutant mice. Dev Brain Res 67:153–160
Author information
Authors and Affiliations
Additional information
This study is dedicated to the memory of the late Professor James A. Norton, Jr (1921–1996) by the coauthors.
Rights and permissions
About this article
Cite this article
Verina, T., Norton, J.A., Sorbel, J.J. et al. Atrophy and loss of dopaminergic mesencephalic neurons in heterozygous weaver mice. Exp Brain Res 113, 5–12 (1997). https://doi.org/10.1007/BF02454137
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02454137