Abstract
Background: This report presents a summary of preclinical data concerning the use of estramustine, an antimicrotubule agent against human glioblastoma cells. The strategy for the investigation of estramustine is predicated on the unique affinity of this agent for microtubule-associated proteins (MAPs).
Methods: A series of laboratory investigations were used to demonstrate antiproliferative effects (MTT assay, colony forming assay, thymidine incorporation), cell cycle synchronization (flow cytometry), intracellular localization of binding sites (immunocytochemistry, electron microscopy), and activity in subcutaneous xenografts of human glioblastoma.
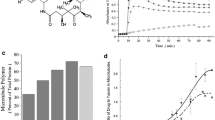
Results: Estramustine has potent in vitro activity against human glioblastoma cells and can enhance the cytotoxic effects of ionizing radiation. Estramustine-binding protein was abundantly expressed in glioblastoma cells and may contribute to the selective effects of estramustine on neoplastic cells. This agent has activity against subcutaneous xenografts of human glioblastoma. Synthesized novel estrogen carbamates also can inhibit proliferation of glioblastoma cells.
Conclusions: Cytoskeletal elements (MAPs) of glioblastoma cells may provide a useful target for therapy with agents like estramustine because of the potent antimitotic effects of this agent and its affinity to a protein that is expressed in glioma cells. These observations have stimulated a search for other estrone carbamates with antimitotic activity that exceeds more conventional antimicrotubule agents.
Similar content being viewed by others
References
Salcman M. Epidemiology and factors affecting survival. In: Apuzzo M, ed.Malignant cerebral gliomas. Park Ridge, Illinois: AANS Pub., 1990:95–110.
Hochberg F, Pruitt A. Assumptions in the radiotherapy of glioblastoma.Neurology 1980;30:907–11.
McComb R, Burger P. Pathologic analysis of primary brain tumors. In:Neurologic clinics. Philadelphia: WB Saunders, 1985.
Daumas-Duport C, Scheithauer B, Kelley P. A histologic and cytologic method for the spatial definition of gliomas.Mayo Clin Proc 1987;62:435–59.
Levin V. Chemotherapy of primary brain tumors. In:Neurologic clinics. Philadelphia: WB Saunders, 1985:855–66.
Schold C, Brent T, von Hoff E, Friedman H, Mitra S, Bigner D, Swenberg J, Klieihues P. O6-alkylguanine-DNA alkyltransferase and sensitivity to procarbazine in human malignant brain tumor xenografts.J Neurosurg 1989;70:573–7.
Chang C, Horton J, Schoenfeld D, Salazer O, Perez-Tamayo R, Kramer S, Weinstein A, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas: a joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study.Cancer 1983;52:997–1007.
Tew K, Glusker J, Hartley-Asp B, Hudes G, Speicher L. Preclinical and clinical perspectives on the use of estramustine as an antimitotic drug.Pharmacol Ther 1992;56:323–39.
Forsgren B, Bjork P. Specific binding of estramustine to prostatic proteins.J Urology 1984;23:34–8.
Stearns M, Jenkins D, Tew M. Dansylated estramustine, a fluorescent probe for studies of uptake and identification of intracellular targets.PNAS 1985;82:8483–7.
Tew KD, Hartley-Asp B. Cytotoxic properties of estramustine unrelated to alkylating and steroid constituents.J Urol 1984;23:28–33.
Tew KD, Stearns ME. Hormone-independent, non-alkylating mechanism of cytotoxicity for estramustine.Urol Res 1987;15:155–60.
Fex H, Hogberg B, Konyves I. Estramustine phosphate—historical review.J Urol 1984;23:4–5.
Tew KD. The mechanism of action of estramustine.Semin Oncol 1983;10:21–6.
Friden B, Wallin M, Deinum J, Prasad V, Luduena R. Effect of estramustine phosphate on the assembly of trypsin-treated microtubules and microtubules reconstituted from purified tubulin with either tau, MAP2 or the tubulin-binding fragment of MAP2.Arch Biochem Biophys 1987;257:123–30.
Kanje M, Deinum J, Wallin M, Ekstrom P, Edstrom A, Hartley-Asp B. Effect of estramustine phosphate on the assembly of isolated bovine brain microtubules and fast axon transport in the frog sciatic nerve.Cancer Res 1985;45:2234–9.
Stearns ME, Tew KD. Estramustine binds to MAP-2 to inhibit microtubule assembly in vitro.J Cell Sci 1988;89:331–42.
von Schoultz E, Lundblad D, Bergh J, Grankvist K, Hendriksson R. Estramustine binding protein and anti-proliferative effect of estramustine in human glioma cell lines.Br J Cancer 1988;58:326–9.
von Schoultz E, Gunnarsson P, Henriksson R. Uptake, metabolism and antiproliferative effect of estramustine phosphate in human glioma cell lines.Anticancer Res 1989;9:1713–6.
Piepmeier J, Keefe D, Weinstein M, Yoshida D, Zielinski J, Lin T, Chen Z, et al. Estramustine and related analogs rapidly and reversibly inhibit DNA synthesis and alter morphology in human glioblastoma cells.Neurosurgery 1993;32:422–31.
Bergenheim A, Gunnarsson P, Edman K, von Schultz E, Hariz M, Henriksson R. Uptake and retention of estramustine and the presence of estramustine binding protein in malignant brain tumors in humans.Br J Cancer 1993;67:358–61.
Yoshida D, Cornell-Bell A, Piepmeier J. Selective antimitotic effects of estramustine correlates with its antimicrotubule properties on glioblastoma and astrocytes.Neurosurgery 1994;34:863–8.
Hartley-Asp B. Estramustine-induced mitotic arrest in two human prostatic carcinoma cell lines DU 145 and PC-3.Prostate 1984;5:93–100.
Yoshida D, Piepmeier J, Weinstein M. Estramustine sensitizes human glioblastoma cells to irradiation.Cancer Res 1994;54:1415–7.
von Schultz E, Bergenheim T, Grankvist K, Henriksson R. Estramustine binding protein in human brain tumor tissue.J Neurosurg 1991;74:962–4.
Forsgren B, Bjork P, Carlstrom K, Gustafsson J, Pousette A, Hogberg B. Purification and distribution of a major protein in rat prostate that binds estramustine, a nitrogen mustard derivative of estradiol-17β.PNAS 1979;76:3149–53.
Suprenant K, Tempero L, Hammer L. Association of ribosomes with in vitro assembled microtubules.Cell Motil Cytoskeleton 1989;14:401–15.
Suprenant K. Microtubules, ribosomes and RNA: evidence for cytoplasmic localization and translational regulation.Cell Motil Cytoskeleton 1993;25:1–9.
Loomis P, Howard T, Castleberry R, Binder L. Identification of nuclear tau isoforms in human neuroblastoma cells.PNAS 1990;87:8422–6.
Walker P, Whitfield J. Cytoplasmic microtubules are essential for the formation of membrane-bound polyribosomes.J Biol Chem 1985;260:765–70.
Schold C, Friedman H, Bigner D. Therapeutic profile of the human glioma line D-54 MG in athymic mice.Cancer Treat Rep 1987;71:849–50.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Piepmeier, J.M., Pedersen, P.E., Yoshida, D. et al. Targeting microtubule-associated proteins in glioblastoma: A new strategy for selective therapy. Annals of Surgical Oncology 3, 543–549 (1996). https://doi.org/10.1007/BF02306087
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02306087