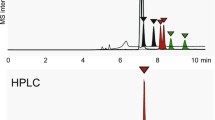
Summary
Water was added to CO2 by saturation to increase the solvation power of the mobile phase in supercritical fluid chromatography. The saturation was performed at a temperature above the boiling point of water (100°C) to increase the amount of water which could be loaded homogeneously into the CO2 (2.5–3.0 mol% water as compared to about 0.25 mol% water at 25°C). A linear composition of water was produced by altering the density of the CO2 during saturation. Modifications to the injector and CO2 transfer lines prevented phase separation as a result of the instrumentation used in capillary supercritical fluid chromatography (SFC). After fitting vapor-liquid equilibria data to pressure, density, and temperature conditions, approximately 2.5–3.0 mol% of water was introduced in a linear gradient at 110°C. The effect of water on SFC performance was evaluated with standard steroid compounds. This paper provides further evidence for the need to examine vapor-liquid equilibria data prior to SFC.
Similar content being viewed by others
References
P. H. van Konynenburg, Ph.D. dissertation, University of California at Los Angeles, 1968.
P. H. van Konynenburg, R. L. Scott, Phil. Trans. Roy. Soc.298, 495 (1980).
S. H. Page, S. R. Sumpter, M. L. Lee, J. Microcol. Sep.4, 91 (1992).
J. S. Rowlinson, F. L. Swinton, Liquids and Liquid Mixtures, 3rd ed., Butterworth Scientific, Boston, Massachusetts, 1969, p. 191.
M. L. McGlashan, A Specialist Report, Chemical Thermodynamics, Vol. 2, The Chemical Society; London, 1978, p. 105.
J. M. Prausnitz, R. N. Lichtenthaler, E. Gomes de Azevedo, Molecular Thermodynamics of Fluid Phase Equilibria, 2nd ed., Prentice-Hall; Englewood Cliffs, New Jersey, 1986, p. 442.
S. H. Page, S. R. Sumpter, S. R. Goates, M. L. Lee, J. Supercrit. Fluids, submitted.
K. K. Liong, P. A. Wells, N. R. Foster, J. Supercrit. Fluids4, 91 (1991).
G. M. Schneider, Fluid Phase Equilibr.10, 141 (1983).
G. M. Schneider, Angew. Chem. Int. Ed. (Engl.)17, 716 (1978).
Yu. V. Tsekhanskaya, Rus. J. Phys. Chem.45, 744 (1971).
I. W. Swaid, G. M. Schneider, Ber. Bunsenges, Phys. Chem.83, 969 (1979).
M. B. Iomtev, Yu. V. Tsekhanskaya, Rus. J. Phys. Chem.38, 485 (1964).
R. Wiebe, Chem. Rev.29, 475 (1941).
C. R. Coan, A. D. King, Jr., J. Amer. Chem. Soc.93, 1837 (1971).
J. E. France, J. M. Snyder, J. W. King, J. Chromatogr.540, 271 (1991).
B. W. Wright, R. D. Smith, J. Chromatogr.355, 367 (1986).
L. A. Allen, T. E. Glass, H. G. Dom, Anal. Chem.60, 390 (1988).
F. O. Geiser, S. G. Yocklovich, S. M. Lurcott, J. W. Guthrie, E. J. Levy, J. Chromatogr.459, 173 (1988).
H. Engelhardt, A. Gross, R. Mertens, M. Petersen, J. Chromatogr.477, 169 (1989).
G. Brunner, Thesis (for Professorship), University of Erlangen, Nürnberg, Germany, 1978.
J. A. Briones, J. C. Mullins, M. C. Thies, B.-U. Kim, Fluid Phase Equilibr.36, 235 (1987).
A. Zawisza, B. Malesińska, J. Chem. Eng. Data26, 388 (1981).
K. Tödheide, E. U. Franck, Z. Physik. Chem. Neue Folge37, 387 (1963).
S. Takenouchi, G. C. Kennedy, Amer. J. Sci.262, 1055 (1964).
R. C. Kong, S. M. Fields, W. P. Jackson, M. L. Lee, J. Chromatogr.289, 105 (1984).
B. W. Wright, P. A. Peaden, M. L. Lee, T. J. Stark, J. Chromatogr.248, 17 (1982).
I. J. Koski, E. D. Lee, I. Ostrovsky, M. L. Lee, Anal. Chem., submitted.
G. Morrison, J. Phys. Chem.85, 759 (1981).
CRC Handbook on Chemistry and Physics, 66th ed., CRC Press; Boca Raton, Florida, pp. D-190, D-213.
J. M. H. Levelt Sengers, W. T. Chen, J. Chem. Phys.56, 595 (1972).
S. H. Page, D. E. Raynie, S. R. Goates, M. L. Lee, D. J. Dixon, K. P. Johnston, J. Microcol. Sep.3, 355 (1991).
S. H. Page, S. R. Goates, M. L. Lee, J. Supercrit. Fluids4, 109 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Page, S.H., Malik, A., Sumpter, S.R. et al. Demonstration of a linear composition gradient during water saturation of CO2 in supercritical fluid chromatography. Chromatographia 37, 93–97 (1993). https://doi.org/10.1007/BF02272195
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02272195