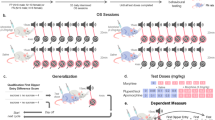
Abstract
Cholecystokinin (CCK) is co-localized with dopamine (DA) in portions of the mesolimbic system, where it may facilitate the function of DA through the CCKA receptor subtype. DA has been implicated in the acquisition of conditioned incentive learning, raising the possibility of a role for endogenous CCK in this learning process. This hypothesis was tested using two complementary behavioral paradigms. Experiment 1 examined the effects of systemic administration of the CCKA receptor selective antagonist, devazepide (0, 0.001, 0.01, 0.1 mg/kg), on the acquisition of conditioned reward. Two novel levers were presented to drug-free animals in a test session; depression of the conditioned reward (CR) lever produced a light-tone stimulus previously paired with food availability while depression of the non-CR lever produced no programmed consequence. Animals receiving vehicle pretreatment in the food-CS conditioning sessions responded more frequently on the CR lever during the test session. However, pre-treatment with devazepide (0.1 mg/kg but not 0.001 or 0.01 mg/kg) in the conditioning sessions blocked the acquisition of conditioned reward. In contrast, experiment 2 showed that the development of conditioned reward was not affected by similar administration of the CCKB selective antagonist, L-365,260 (0, 0.001, 0.01 or 0.1 mg/kg). The possibilities that devazepide (0.1 mg/kg) impaired the development of conditioned reward by decreasing the amount of food consumed or by inducing a conditioned taste aversion to the food were ruled out in experiments 3 and 4. The effects of devazepide on the acquisition of conditioned activity induced by amphetamine were assessed in experiment 5. During four conditioning sessions, rats received devazepide (0, 0.001, 0.01, 0.1 or 1.0 mg/kg) treatment prior to amphetamine-environment pairings. The conditioned activity effect was demonstrated if on the subsequent drug-free test day the environment alone elicited increased locomotion. Devazepide (0.1 or 1.0 mg/kg) attenuated the development of conditioned activity. Together, these results provide converging evidence that intact CCKA function may be necessary for the development of conditioned incentive learning.
Similar content being viewed by others
References
Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ (1981) The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res 212:51–57
Beninger RJ (1991) Receptor subtype-specific dopamine agonists and antagonists and conditioned behavior. In: Willner P, Scheel-Kruger J (eds) The mesolimbic dopamine system: from motivation to action. Wiley, New York, pp 273–299
Beninger RJ, Hahn BL (1983) Pimozide blocks establishment but not expression of amphetamine-produced environment-specific conditioning. Science 220:1304–1306
Beninger RJ, Phillips AG (1980) The effects of pimozide on the establishment of conditioned reinforcement. Psychopharmacology 68:147–153
Beninger RJ, Hoffman DC, Mazurksi EJ (1989) Receptor subtype-specific dopaminergic agents and conditioned behavior. Neurosci Biobehav Rev 13:113–122
Chang RSL, Lotti VI (1986) Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci USA 83:4923–4926
Crawley JN (1992) Subtype-selective cholecystokinin receptor antagonists block cholecystokinin modulation of dopamine-mediated behaviors in the rat mesolimbic pathway. J Neurosci 12:3380–3393
Crawley JN, Corwin RL (1994) Biological actions of cholecystokinin. Peptides 15:731–755
Crawley JN, Hommer DW, Skirboll LR (1985) Topographical analysis of nucleus accumbens sites at which cholecystokinin potentiates the dopamine induced hyperlocomotion in the rat. Brain Res 335:337–341
Crawley JN, Stivers JA, Hommer DW, Skirboll LR, Paul SM (1986) Antagonists of central and peripheral behavioral effects of cholecystokinin octapeptide. J Pharmacol Exp Ther 236:320–330
Dockray GJ (1976) Immunochemical evidence of cholecystokinin-like peptides in the brain. Nature 264:568–570
Dourish CT, Hill DR (1987) Classification and function of CCK receptors. Trends Pharmacol Sci 8:207–208
Emson PC, Marley PD (1983) Cholecystokinin and vasoactive intestinal polypeptide. In: Iversen L, Iversen SD, Snyder SH (eds) Handbook of psychopharmacology, 16th edn. Plenum, New York, pp 255–306
Fallon JH, Seroogy KB (1985) The distribution and some connections of cholecystokinin neurons in the rat brain. Ann NY Acad Sci 448:121–132
Hewson G, Leighton GE, Hill RG, Hughes J (1988) The CCK receptor antagonist L364,718 increases food intake in the rat by attenuation of the action of endogenous CCK. Br J Pharmacol 93:79–84
Higgins GA, Nguyen P, Sellers EM (1991) Blockade of morphine place conditioning by the CCKA receptor antagonist devazepide. Eur J Pharmacol 197:229–230
Higgins GA, Nguyen P, Sellers EM (1992) Morphine place conditioning is differentially affected by CCKA and CCKB receptor antagonists. Brain Res 572:208–215
Higgins GA, Joharchi N, Wang Y, Corrigall WA, Sellers EM (1994a) The CCKA receptor antagonist devazepide does not modify opioid self-administration or drug discrimination: comparison with the dopamine antagonist haloperidol. Brain Res 640:246–254
Higgins GA, Sills TL, Tomkins DM, Sellers EM, Vaccarino FJ (1994b) Evidence for the contribution of CCKB receptor mechanisms to individual differences in amphetamine-induced locomotion. Pharmacol Biochem Behav 48:1019–1024
Hoffman DC, Beninger RJ (1985) The effects of pimozide on the establishment of conditioned reinforcement as a function of the amount of conditioning. Psychopharmacology 87:454–460
Hokfelt T, Skirboll L, Rehfeld JF, Goldstein M, Markey K, Dann O (1980) A subpopulation of mesencephalic dopamine neurons projecting to limbic areas contain cholecystokinin-like peptide: evidence from immunohistochemistry combined with retrograde tracing. Neuroscience 5:2093–2124
Hokfelt T, Holets VR, Staines W, Meister B, Melander T, Schalling M, Schultzberg M, Freedman J, Bjorkland H, Olson L, et al. (1986) Co-existence of neuronal messengers: an overview. Prog Brain Res 68:33–70
Ingram SM, Krause RG, Baldino F, Skeen LC, Lewis ME (1989) Neuronal localization of cholecystokinin mRNA in the rat brain by using in situ hybridization histochemistry. J Comp Neurol 287:260–272
Innis RB, Snyder SH (1980) Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci USA 77:6917–6921
Jiang LH, Kasser RJ, Wang RY (1988) Cholecystokinin antagonist lorglumide reverses chronic haloperidol-induced effects on dopamine neurons. Brain Res 473:165–168
Josselyn SA, Vaccarino FJ (1995) Interactions of CCKB receptors with amphetamine in responding for conditioned rewards Peptides 16:959–964
Khosla S, Crawley JN (1988) Potency of L-364,718 as an antagonist of the behavioral effects of peripherally administered cholecystokinin. Life Sci 42:153–159
Lemaire M, Piot O, Roques BP, Bohme GA, Blanchard JC (1992) Evidence for an endogenous cholecystokinergic balance in social memory. Neuroreport 3:929–932
Lotti VJ, Chang RSL (1989) A potent and selective non-peptide gastrin antagonist and brain CCK receptor (CCKB) ligand: L-365,260. Eur J Pharmacol 162:273–280
Marshall FH, Barnes S, Hughes J, Woodruff GN, Hunter JC (1991) Cholecystokinin modulates the release of dopamine from the anterior and posterior nucleus accumbens by two different mechanisms. J Neurochem 56:917–922
Massey BW, Vanover KE, Woolverton WL (1994) Effects of cholecystokinin antagonists on the discriminative stimulus effects of cocaine in rats and monkeys. Drug Alcohol Depend 34:105–111
Mazurksi EJ, Beninger RJ (1991) Effects of selective drugs for dopaminergic D1 and D2 receptors on conditioned locomotion in rats. Psychopharmacology 105:107–112
Minabe Y, Ashby CRJ, Wang RY (1991) The CCKA receptor antagonist devazepide but not the CCKB receptor antagonist L-365,260 reverses the effects of chronic clozapine and haloperidol on midbrain neurons. Brain Res 549:151–154
Moran TH, Robinson PH, Goldrich MS, McHugh PR (1986) Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res 362:175–179
Nachman M, Ashe JH (1973) Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiol Behav 10:73–78
Phillips GD, LeNoury J, Wolterink G, Donsellar-Wolterink J, Robbins TW, Everitt BJ (1993) Cholecystokinin-dopamine interactions within the nucleus accumbens in the control over behavior by conditioned reinforcement. Behav Brain Res 55:223–231
Pickens RW, Crowder WF (1967) Effects of CS-US interval on conditioning of drug response, with assessment of speed of conditioning. Psychopharmacology 11:88–94
Seroogy KB, Brecha N, Gall C (1985) Distribution of CCK-like immunoreactivity in the rat main olfactory bulb. J Comp Neurol 239:373–383
Skirboll LF, Grace AA, Hommer DW, Rehfeld JF, Goldstein M, Hokfelt T, Bunney BS (1981) Peptide-monoamine coexistence: studies of the actions of CCK-like peptides on the electrical activity of midbrain DA neurons. Neuroscience 6:2111–2114
Spealman RD (1992) Failure of cholecystokinin antagonists to modify the discriminative stimulus effects of cocaine. Pharmacol Biochem Behav 43:613–616
Stewart J, Vezina P (1987) Environment-specific enhancement of the hyperactivity induced by systemic or intra-VTA morphine injections in rats pre-exposed to amphetamine. Psychobiology 15:144–153
Taylor JR, Robbins TW (1984) Enhanced behavioral control by conditioned reinforcers following microinjection ofd-amphetamine into the nucleus accumbens. Psychopharmacology 84:405–412
Vaccarino FJ (1994) Nucleus accumbens dopamine-CCK interactions in psychostimulant reward and related behaviors. Neurosci Biobehav Rev 18:207–214
Vaccarino FJ, Rankin J (1989) Nucleus accumbens cholecystokinin (CCK) can either attenuate or potentiate amphetamine-induced locomotor activity; evidence for rostral-caudal differences in accumbens CCK function. Behav Neurosci 103:831–836
Vanderhaeghen JJ, Signeau JC, Gepts W (1975) New peptide in the vertebrate CNS reacting with gastrin antibodies. Nature 257:604–605
Vezina P, Stewart J (1989) The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res 499:108–120
Wang RY, Hu XT (1986) Does cholecystokinin potentiate dopamine action in the nucleus accumbens? Brain Res 380:363–367
Wank SA, Pisegna JR, DeWeerth A (1992) Brain and gastrointestinal cholecystokinin receptor family: structure and functional expression. Proc Natl Acad Sci USA 89:8670–8691
White FJ, Wang RY (1984) Interactions of CCK octapeptide and dopamine on nucleus accumbens neurons. Brain Res 300:161–166
Winer BJ (1971) Statistical principles in experimental design. McGraw Hill, New York
Zaborsky L, Alheid GF, Beinfeld CM, Eiden LE, Heimer L, Palkovitz M (1985) Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neurosci 14:427–453
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Josselyn, S.A., Franco, V.P. & Vaccarino, F.J. Devazepide, a CCKA receptor antagonist, impairs the acquisition of conditioned reward and conditioned activity. Psychopharmacology 123, 131–143 (1996). https://doi.org/10.1007/BF02246170
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02246170