Summary
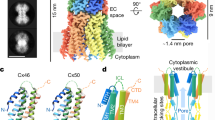
Membranes rich in junction complexes were prepared from bovine lens, and the fragments of the membranes were reconstituted into proteoliposomes with a large excess of phosphatidylcholine and dicetylphosphate. The osmotic swelling behavior of these liposomes showed that the lens junction membranes contributed protein components that produced channels with a nominal diameter of 1.4 nm. Most preparations of lens junctions produced rates of osmotic swelling much slower than those found in proteoliposomes containing equivalent amounts ofEscherichia coli porin, and we discuss several possible explanations for this observation.
Similar content being viewed by others
References
Bernardini, G., Peracchia, C. 1981. Gap junction crystallization in lens fibers after an increases in cell calcium.Invest. Ophthalmol. 21:291–299
Brockhuyse, R.M., Kuhlman, E.D. 1978. Lens membrane: IV. Preparative isolation and characterization of the membranes and various membrane proteins from calf lens.Exp. Eye Res. 26:305–320
Brockhuyse, R.M., Kuhlman, E.D., Bijvelt, J., Verkleij, A.J., Ververgaert, P.H.T. 1978. Lens membranes: III. Freeze fracture morphology and composition of bovine lens fibre membranes in relation to ageing.Exp. Eye Res. 26:147–156
Caspar, D.L.D., Goodenough, D.A., Makowski, L., Phillips, W.C. 1977. Gap junction structures: I. Correlated electron microscopy and X-ray diffraction.J. Cell Biol. 74:605–628
Cohen, A.I. 1965. The electron microscopy of the normal human lens.Invest. Ophthalmol. 4:443–446
Colombini, M. 1979. A candidate for the permeability pathway of the outer mitochondrial membrane.Nature (London) 279:643–645
Costello, M.J., McIntosh, T.-J., Robertson, J.D. 1984. Square array fiber cell membrane in mammalian lens.In: Proceedings 42nd Meeting of Electron Microscopy Society of America. D.W. Dailey, editor, pp. 126–129. San Francisco Press, Sand Francisco
FitzGerald, P.G., Bok, D., Horwitz, J. 1983. Immunocytochemical localization of the main intrinsic polypeptide (MIP) in ultrathin frozen sections of rat lens.J. Cell Biol. 97:1491–1499
French, D., Levine, M., Pazur, J.H. 1949. Studies on the Schardinger dextrins: II. Preparation and properties of amyloheptaose.J. Am. Chem. Soc. 71:356–358
Goodenough, D.A. 1974. Bulk isolation of mouse hepatocyte gap junctions.J. Cell Biol. 61:557–563
Goodenough, D.A. 1979. Lens gap junctions: A structural hypothesis for nonregulated low-resistance intercellular pathways.Invest. Ophthalmol. 18:1104–1122
Hertzberg, E.L., Anderson, D.J., Friedlander, M., Gilula, N.B. 1982. Comparative analysis of the major polypeptides from liver gap junctions and lens fiber junctions.J. Cell. Biol. 92:53–59
Kuszak, J.R., Rae, J.L., Pauli, B.U., Weinstein, R.S. 1982. Rotary replication of lens gap junctions.J. Ultrastruct. Res. 81:249–256
Loewenstein, W.R. 1981. Junctional intercellular communication: The cell-to-cell membrane channel.Physiol. Rev. 61:829–913
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. 1951. Protein measurement with the Folin phenol reagent.J. Biol. Chem. 193:265–275
Lugtenberg, B., Meijers, J., Peters, R., Hoek, P. van der, Alphen, L. van 1975. Electrophotretic resolution of the ‘major outer membrane protein’ ofEscherichia coli K12 into four bands.FEBS Lett. 58:254–258
Makowski, L., Caspar, D.L.D., Phillips, W.C., Goodenough, D.A. 1977. Gap junction structures: II. Analysis of the X-ray diffraction data.J. Cell Biol. 74:629–645
Nakae, T. 1976. Outer membrane ofSalmonella. Isolation of protein complex that produces transmembranes channels.J. Biol. Chem. 251:2176–2178
Nikaido, H., Rosengerg, E.Y. 1983. Porin channels inEscherichia coli: Studies with liposomes reconstituted from purified proteins.J. Bacteriol. 153:241–252
Peracchia, C. 1977. Gap junctions: Structural changes after uncoupling procedures.J. Cell Biol. 72:628–641
Peracchia, C. 1978. Structural correlates of gap junction permeation.Int. Rev. Cytol. 66:81–145
Peracchia. C., Girsh, S.J. 1984. Calmodulin-mediated gating of lens gap junction channels in vesicles.In: Proceedings 42nd Meeting of Electron Microscopy Society of America. G.W. Dailey, editor. pp. 134–137. San Francisco Press, San Francisco
Peracchia, C., Peracchia, L.L. 1980. Gap junction dynamics: Reversible effects of divalent cations.J. Cell Biol. 87:708–718
Renkin, E.M. 1954. Filtration, diffusion, and molecular sieving through porous cellulose membranes.J. Gen. Physiol. 38:225–243
Rose, B., Loewenstein, W.R. 1975. Permeability of cell junction depends on local cytoplasmic calcium activity.Nature (London) 254:250–252
Schindler, H., Rosenbusch, J.P. 1978. Matrix protein fromE. coli outer membrane forms voltage-controlled channels in lipid bilayers.Proc. Natl. Acad. Sci. USA 75:3751–3755
Schwarzmann, G., Wiegandt, H., Rose, B., Zimmerman, A., Ben-haim, D., Loewenstein, W.R. 1981. Diameter of the cell-to-cell junctional membrane channels as probed with neutral molecules.Science 213:551–553
Simon, S.A., Zampighi, G., McIntosh, T.J., Costello, J., Ting-Beall, H.P., Robertson, J.D. 1982. The structure of junctions between lens fiber cells.Biosci. Rep. 2:333–341
Unwin, P.N.T., Ennis, P.D. 1983. Calcium-mediated changes in gap junction structure: Evidence from the low angle X-ray pattern.J. Cell Biol. 97:1459–1465
Unwin, P.N.T., Zampighi, G. 1980. Structure of the junction between communicating cells.Nature (London) 283:545–549
Yoshimura, F., Zalman, L.S., Nikaido, H. 1983. Purification and properties ofPseudomonas aeruginosa porin.J. Biol. Chem. 258:2308–2314
Zalman, L.S., Nikaido, H., Kagawa, Y. 1980. Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels.J. Biol. Chem. 255:1771–1774
Zampighi, G., Simon, S.A., Robertson, J.D., McIntosh, T.J., Costello, M.J. 1982. On the structural organization of isolated bovine lens fiber junctions.J. Cell Biol. 93:175–189
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nikaido, H., Rosenberg, E.Y. Functional reconstitution of lens gap junction proteins into proteoliposomees. J. Membrain Biol. 85, 87–92 (1985). https://doi.org/10.1007/BF01872008
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01872008