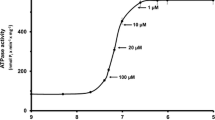
Summary
Calsequestrin was identified in the isolated sarcoplasmic reticulum from skeletal muscle of three mammalian species (man, rat and rabbit) and from frog and chicken muscle, using electrophoretic and immunoblot techniques. It was further characterized in sarcoplasmic reticulum protein mixtures and at several stages of purification, following extraction with EDTA.
We found extensive similarities in apparent molecular weight values, Stains All staining properties and in Cleveland's peptide maps, between mammalian calsequestrins, and no detectable difference within a species between fast and slow muscle. Human calsequestrin, with an apparent molecular weight of 60 000 when measured at alkaline pH and of 41 000 when measured at neutral pH, appears to be the smallest in size. Frog calsequestrin, although weakly cross-reactive with rabbit calsequestrin and having a relatively higher apparent molecular weight at alkaline pH (72 000), shares several significant properties with mammalian calsequestrins. It bound calcium with a high capacity (1300 nmol per mg protein), it contained about 32% acidic amino acid residues and focused at closely similar pI values. We observed the formation of a complex with Stains All absorbing maximally at 535 nm, rather than at 600 nm, and an even more marked shift in apparent molecular weight at neutral pH.
We found distinct differences in the case of chicken calsequestrin, in addition to those previously reported. It is a highly acidic, calcium-precipitable protein, but its amino acid composition is contradistinguished by a higher ratio of glutamate to aspartate and its rate of electrophoretic mobility is minimally affected by changes in pH. It stained deep bluish with Stains All after gel electrophoresis and yielded a protein-dye complex in aqueous solution, absorbing maximally at 560 nm, and finally, it bound fluorescent Concanavalin A.
Similar content being viewed by others
References
Ashhurst, D. E. (1969) The fine structure of pigeon breast muscle.Tiss. Cell 1, 485–96.
Bean, R. C. M., Sheperd, W. C., Kay, R. E. &Walwick, E. R. (1965) Spectral changes in a cationic dye due to interactions with macromolecules. III. Stoichiometry and mechanism of the complexing reaction.J. phys. Chem. 69, 4368–79.
Biral, D., Damiani, E., Margreth, A. &Scarpini, E. (1984) Myosin subunit composition in human developing muscle.Biochem. J. 224, 923–31.
Campbell, K. P., Franzini-Armstrong, C. &Shamoo, A. E. (1980) Further characterization of light and heavy sarcoplasmic reticulum vesicles. Identification of the ‘sarcoplasmic reticulum feet’ associated with heavy sarcoplasmic reticulum vesicles.Biochim. biophys. Acta 602, 97–116.
Campbell, K. P. &MacLennan, D. H. (1981) Purification and characterization of the 53 000-dalton glycoprotein from sarcoplasmic reticulum.J. biol. Chem. 256, 4626–32.
Campbell, K. P., MacLennan, D. H., Jorgensen, A. O. &Mintzer, M. C. (1983a) Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53 000 dalton glycoprotein.J. biol. Chem. 258, 1197–204.
Campbell, K. P., MacLennan, D. H. &Jorgensen, A. O. (1983b) Staining of the Ca-binding proteins, calsequestrin, calmodulin, troponin C, and S-100 with the cationic dye ‘Stains All’.J. biol. Chem. 258, 11267–73.
Cleveland, D. W., Fischer, S. G., Kirschner, M. W. &Laemmli, U. K. (1977) Peptide mapping by limited proteolysis in sodium dodecyl sulphate by gel electrophoresis.J. biol. Chem. 252, 1102–6.
Damiani, E., Betto, R., Salvatori, S., Volpe, P., Salviati, G. &Margreth, A. (1981) Polymorphism of sarcoplasmic reticulum adenosine triphosphatase of rabbit skeletal muscle.Biochem. J. 197, 245–8.
Duggan, P. F. &Martonosi, A. (1970) IX. The permeability of sarcoplasmic reticulum membranes.J. gen. Physiol. 56, 147–57.
Eastwood, A. B., Franzini-Armstrong, C. &Peracchia, C. (1982) Structure of membranes in crayfish muscle: comparison of phasic and tonic fibres.J. Musc. Res. Cell Motility 3, 273–94.
Forbes, M. S. &Sperelakis, N. (1980) Membrane systems in skeletal muscle of the lizardAnolis carolinensis.J. Ultrastruct. Res. 73, 245–61.
Franzini-Armstrong, C. (1977) The comparative structure of intracellular junctions in striated muscle fibers. InPathogenesis of Human Muscular Dystrophies (edited byRowland, L. P.), pp. 612–25. Amsterdam: Excerpta Medica.
Franzini-Armstrong, C. &Nunzi, G. (1983) Junctional feet and particles in the triads of a fast-twitch muscle fibre.J. Musc. Res. Cell Motility 4, 233–52.
Hager, D. A. &Burgess, R. R. (1980) Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity.Analyt. Biochem. 109, 76–86.
Houmard, J. &Drapeau, G. R. (1982) Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds.Proc. natn. Acad. Sci. U.S.A. 69, 3506–9.
Ikemoto, N., Bhatnagar, G. M. &Gergely, J. (1971) Fractionation of solubilized sarcoplasmic reticulum.Biochem. Biophys. Res. Commun. 44, 1510–7.
Jorgensen, A. O. &Campbell, K. P. (1984) Evidence for the presence of calsequestrin in two structurally different regions of myocardial sarcoplasmic reticulum.J. Cell Biol. 98, 1597–602.
Jorgensen, A. O., Kalnins, V. &MacLennan, D. H. (1979) Localization of sarcoplasmic reticulum proteins in rat skeletal muscle by immunofluorescence.J. Cell Biol. 80, 372–84.
Jorgensen, A. O., Shen, A. C. -Y. &Campbell, K. P. (1985) Ultrastructural localization of calsequestrin in adult rat atrial and ventricular muscle cells.J. Cell Biol. 101, 257–68.
Jorgensen, A. O., Shen, A. C. Y., Campbell, K. P. &MacLennan, D. H. (1983) Ultrastructural localization of calsequestrin in rat skeletal muscle by immunoferritin labeling of ultrathin frozen sections.J. Cell Biol. 97, 1573–81.
Junker, J. &Sommer, J. (1979) Calsequestrin localization in rabbit and frog skeletal muscle by immunofluorescence.J. Cell Biol. 83, 384a.
Kay, R. E., Walwick, E. R. &Gifford, C. K. (1964) Spectral changes in a cationic dye due to interaction with macromolecules. I. Behaviour of dye alone in solution and the effect of added macromolecules.J. phys. Chem. 68, 1896–906.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature, Lond. 227, 680–4.
Lowry, O. H., Rosebrough, N. J., Farr, A. L. &Randall, R. J. (1951) Protein measurements with the Folin phenol reagent.J. biol. Chem. 193, 265–75.
MacLennan, D. H. (1974) Isolation of a second form of calsequestrin.J. biol. Chem. 249, 980–4.
MacLennan, D. H. &Wong, P. T. S. (1971) Isolation of a calcium-sequestering protein from sarcoplasmic reticulum.Proc. natn. Acad. Sci. U.S.A. 68, 1231–5.
MacLennan, D. H., Campbell, K. P. &Reithmeier, R. A. F. (1983) Calsequestrin. InCalcium and Cell Function (edited byMartonosi, A.), Vol 4, pp. 151–73. New York: Academic Press.
Maurer, A., Tanaka, M., Ozawa, T. &Fleischer, S. (1985) Purification and crystallization of the calcium binding protein of sarcoplasmic reticulum from skeletal muscle.Proc. natn. Acad. Sci. U.S.A. 82, 4036–40.
Meissner, G. (1975) Isolation and characterization of two types of sarcoplasmic reticulum vesicles.Biochim. biophys. Acta 389, 51–68.
Michalak, M., Campbell, K. P. &MacLennan, D. H. (1980) Localization of the high affinity calcium binding protein and an intrinsic glycoprotein in sarcoplasmic reticulum membranes.J. biol. Chem. 255, 1317–26.
O'Farrell, P. H. (1975) High resolution two-dimensional electrophoresis of proteins.J. biol. Chem. 250, 4007–21.
Page, S. G. (1964) Structure and some contractile properties of fast and slow muscles of the chicken.J. Physiol., Lond. 205, 131–45.
Sabbadini, R. A. &Okamoto, V. (1983) The distribution of ATPase activities in purified transverse tubular membranes.Archs Biochem. Biophys. 223, 107–19.
Saito, A., Seiler, S., Chu, A. &Fleischer, S. (1984) Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle.J. Cell Biol. 99, 875–85.
Salviati, G., Volpe, P., Salvatori, S., Betto, R., Damiani, E., Margreth, A. &Pasquali-Ronchetti, I. (1982) Biochemical heterogeneity of skeletalmuscle microsomal membranes. Membrane origin, membrane specificity and fiber types.Biochem. J. 202, 289–301.
Somlyo, A. V., Gonzales-Serratos, H., Shuman, H., McClellan, G. &Somlyo, A. P. (1981) Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study.J. Cell Biol. 90, 577–94.
Sommer, J. R. (1982) The anatomy of the sarcoplasmic reticulum in vertebrate skeletal muscle: its implications for excitation-contraction coupling.Z. Naturf. 37c, 665–8.
Spray, T. L., Waugh, R. A. &Sommer, J. R. (1974) Peripheral couplings in adult vertebrate skeletal muscle.J. Cell Biol. 62, 223–7.
Towbin, H., Staehelin, T. &Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications.Proc. natn. Acad. Sci. U.S.A. 76, 4350–4.
Volpe, P., Biral, D., Damiani, E. &Margreth, A. (1981) Characterization of human muscle myosin with respect to the light chains.Biochem. J. 195, 251–8.
Weber, K. &Osborn, M. (1969) The reliability of molecular weight determination by dodecyl sulphate polyacrylamide gel electrophoresis.J. biol. Chem. 244, 4406–10.
White, D. M., Colwyn, R. T. &Denborough, M. A. (1983) A novel method for the isolation of calsequestrin from porcine skeletal muscle sarcoplasmic reticulum.Biochim. biophys. Acta 744, 1–6.
Yap, J. L. &MacLennan, D. H. (1976) Characterization of the adenosinetriphosphatase and calsequestrin isolated from sarcoplasmic reticulum of normal and dystrophic chickens.Can. J. Biochem. 54, 670–3.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Damiani, E., Salvatori, S., Zorzato, F. et al. Characteristics of skeletal muscle calsequestrin: comparison of mammalian, amphibian and avian muscles. J Muscle Res Cell Motil 7, 435–445 (1986). https://doi.org/10.1007/BF01753586
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01753586