Summary
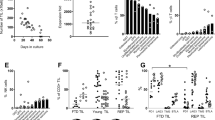
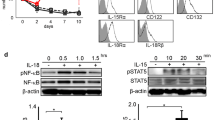
Outgrowth of tumor-infiltrating lymphocytes (TIL) from the human primary brain tumor glioblastoma multiforme was achieved by OKT3 initiation (10 ng/ml), followed by sustained expansion by interleukin-2 (IL-2; 200 U/ml). Tumor-infiltrating lymphocyte (TIL) initiation by this process was performed in parallel with the standard “IL-2-only” method. Of ten tumors, seven yielded TIL in response to OKT3/IL-2, whereas only three of these seven grew after initiation with IL-2 alone. On the basis of cell doubling times, at least 60 doublings, resulting in (hypothetically) up to 1023 TIL from as few as 2 × 105 cells in tumor suspensions, could be achieved using OKT3/IL-2. OKT3-initiated TIL proliferated in culture for as long as 288 days, although senescence of some cultures occurred at as early as 73 days. Significant heterogeneity of lymphocytes infiltrating the fresh tumors and heterogeneity of resultant TIL phenotype and function were apparent, yet several common trends were noted. In all cases after OKT3 initiation, significant net growth was not apparent until approximately 14 days. In contrast, in the three samples that grew in response to IL-2 alone, log-phase growth was always observed earlier. During the early phase of the cultures, all TIL expressed some killing activity toward a broad spectrum of tumors, including the autologous tumor. No consistent preference of TIL for lysis of autologous tumor was observed. Glioblastoma multiforme TIL cultures contained a mixture of CD8+ and CD4+ cells, with few CD16+ or NKH-1+. Of the six TIL examined in detail for population phenotype in relationship to time in culture, four eventually became exclusively CD4+. Further analysis of these CD4+ TIL indicated that all were of the helper-inducer class, 4B4+ and 2H4−. Concurrent with the decline in CD8+ cells, a decline in the cytolytic activity of these TIL cultures occurred. Furthermore, in two TIL that remained CD8+, a decline in the cytolytic activity also occurred. Therefore, loss of killing activity was not merely a reflection of the major cell phenotype changes. These results indicate that the OKT3/IL-2 process provides an alternative to IL-2 alone for TIL initiation and growth, as well as providing a novel system for further analysis of tumorderived lymphocyte and accessory cell functional potential.
Similar content being viewed by others
References
Anderson PM, Blazar BR, Bach FH, Ochoa AC (1989) Anti-CD3+IL-2 stimulated murine killer cells. In vitro generation and in vivo antitumor activity. J Immunol 142: 1383–1394
Belledegrum A, Muul LM, Rosenberg SA (1988) Interleukin 2 expanded tumor-infiltrating lymphocytes in human renal cell cancer: isolation, characterization, and antitumor activity. Cancer Res 48: 206–214
Brooks C, Holscher M (1987) Cell surface molecules involved in NK recognition by cloned cytotoxic T lymphocytes. J Immunol 138: 1331–1338
Brooks WH, Markesbery WR, Gupta GD, Roszman TL (1978) Relationship of lymphocyte invasion and survival of brain tumor patients. Ann Neurol 4: 219–224
Brooks CG, Holscher M, Urdal D (1985) Natural killer activity in cloned cytolytic T lymphocytes: regulation by interleukin 2, interferon, and specific antigen. J Immunol 135: 1145–1158
Farmer J-P, Antel JP, Freedman M, Cashman NR, Rode H, Villemure J-G (1989) Characterization of lymphoid cells isolated from human gliomas. J Neurosurg 71: 528–533
Fernandez-Cruz E, Woda BA, Feldman JD (1980) Elimination of syngeneic sarcomas in rats by a subset of T lymphocytes. J Exp Med 823: 8417–8424
Fierz W, Endler B, Reske K, Wekerle H, Fontana A (1985) Astrocytes as antigen-presenting cells: I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol 134: 3785–3793
Fontana A, Hengartner H, De Tribolet N, Weber F (1984) Gliobastoma cells release interleukin-1 and factors inhibiting interleukin-2 mediated effects. J Immunol 132: 1837–1844
George RE, Loudon WG, Moser RP, Brunner JM, Steck PA, Grimm EA (1988) In vitro cytolysis of primitive neuroectoderma tumors of the posterior fossa (meduloblastoma) by lymphokine-activated killer cells. J Neurosurg 69: 403–409
Grimm, EA, Crump III WL, Durret A, Hester JP, Lagoo-Deenadalayan S, Owen-Schaub LB (1988) TGF-beta inhibition of the in vitro induction of lymphokine-activated killing activity is reversible. Cancer Immunol Immunother 27: 53–62
Grimm EA, Moser RP, Blacklock JB, Loudon W, Carinhas J (1990) Interleukin-2 and cytotoxic lymphocyte therapy of primary and metastatic brain tumors. In: Johanssen B, Owrhan C, Widner H (eds) Pathophysiology of the BBB. Elsevier, Amsterdam, 527–538
Gullberg M, Smith KA (1986) Regulation of T cell autocrine growth. T4+ cells become refractory to interleukin 2. J Exp Med 163: 270–284
Heo DS, Whiteside TL, Johnson JT, Chen K, Barnes EL, Herberman RB (1987) Long-term interleukin 2-dependent growth and cytotoxic activity of tumor-infiltrating lymphocytes from human squamos cell carcinomas of the head and neck. Cancer Res 47: 6353–6362
Hersey P, MacDonald M, Schibeci S, Burns C (1986) Clonal analysis of cytotoxic T lymphocytes (CTL) against autologous melanoma: classification based on phenotype, specificity and inhibition by monoclonal antibodies to T cell structures. Cancer Immunol Immunother 22: 15–21
Itoh K, Tilden AB, Balch CM (1986) Interleukin 2 activation of cytotoxic T-lymphocytes infiltrating human metastatic melanomas. Cancer Res 46: 3011–3017
Jacobs SK, Wilson DJ, Kornblith PL, Grimm EA (1986) Interleukin-2 and autologous lymphokine activated killer (LAK) cells in the treatment of malignant glioma: preliminary report. J Neurosurg 372: 386–389
Kawakami Y, Custer MC, Rosenberg SA, Lotze MT (1989) IL-4 regulates IL-2 induction of lymphokine-activated killer activity from human lymphocytes. J Immunol 142: 3452–3461.
Kradin RL, Boyle LA, Preffer FI, et al. (1987) Tumor-derived interleukin-2 dependent lymphocytes in adoptive immunotherapy of lung cancer. Cancer Immunol Immunother 24: 76–85
Kuppner MC, Hamou MF, De Tribolet N (1988) Immunohistologica and functional analyses of lymphoid infiltrates in human glioblastomas. Cancer Res 48: 6926–6932
Miescher S, Whiteside TL, De Tribolet N, Von Fliedner V (1988) In situ characterization, clonogenic potential and antitumor cytolytic activity of T-lymphocytes infiltrating human brain cancers J Neurosurg 68: 438–448
Muul LM, Spiess PL, Director EP, Rosenberg SA Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol 138: 989–998
Moy PM, Holmes EC, Golub SH (1985) Depression of natural killer cytotoxic activity in lymphocytes infiltrating human pulmonar tumors. Cancer Res 45: 57–60
Ochoa AC, Hasz DE, Rezonzew R, Anderson PM, Bach FH (1989) Lymphokine-activated killer activity in long-term cultures with anti-CD3 plus interleukin 2: identification and effector subsets. Cancer Res 49: 963–968
Owen-Schaub LB, Gutterman JU, Grimm EA (1988) Synergy of TNF in the activation of human cytotoxic lymphocytes. Tumor necrosis factor alpha is synergistic with interleukin-2 in the generation of human lymphokine-activated killer cell cytotoxicity. Cancer Res 48: 788–792
Phillips JH, Takeshita T, Sugamura K, Lanier LL (1989) Activation of natural killer cells via the p75 interleukin 2 receptor. J Exp Med 170: 291–296
Ridley A, Cavanagh JB (1971) Lymphocyte infiltration in gliomas. Evidence of possible host resistance. Brain 94: 117–124
Robbins JC, Nicolson GL (1983) Surfaces of normal and transformed cells. In: Becker FF (ed) Cancer: a comprehensive treatise, vol 4. Plenum, New York, p 3. 1983
Rosenberg SA, Packard BS, Aebersold PM, et al. (1988) Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. New Engl J 319: 1676–1680
Sawamura Y, Abe H, Aida T, Hosokawa M, Kobayashi H (1988) Isolation and in vitro growth of glioma-infiltrating lymphocytes and an analysis of their surface phenotypes. J Neurosurg 69: 745–750
Sawamura Y, Hosokawa M, Kuppner MC, et al. (1989) Antitumor activity and surface phenotypes of human glioma-infiltrating lymphocytes after in vitro expansion in the presence of interleukin 2. Cancer Res 49: 1843–1849
Schoof DD, Selleck CM, Massaro AF, Jung S-F, Eberlein TJ (1990) Activation of tumor infiltrating lymphocyte by monoclonal antibodies directed to the CD3 complex. Cancer Res 50: 1138
Sedgwick J, Brostoff S, Mason D (1987) Experimental allergic encephalomyelitis in the absence of a classical delayed-type hypersensitivity reaction. J Exp Med 165: 1058–1075
Takeshita T, Goto Y, Tada K, Nagata K, Asao H, Sugamura K (1989) Monoclonal antibody defining a molecule possibly identical to the p 75 subunit of interleukin 2 receptor. J Exp Med 169: 1323–1332
Von Hanwehr RI, Hofman FM, Taylor CR, Appuzo MLJ (1984) Mononuclear lymphoid populations infiltrating the microenvironment of primary CNS tumors. J Neurosurg 60: 1138–1147
Whiteside TL, Heo DS, Takagi S, Johnson JT, Iwatsuki S, Herberman RB (1988) Cytolytic antitumor effector cells in long term cultures of human tumor-infiltrating lymphocytes in recombinant interleukin 2. Cancer Immunol Immunother 26: 1–10
Whiteside TL, Miescher S, Hurlimann J, Moretta L, Von Fliedner V (1986) Separation, phenotyping and limiting dilution analysis of T-lymphocytes infiltrating human solid tumors. Int J Cancer 37: 803–811
Wolf JA, Carinhas J, Grimm EA (1989) Flow cytometric conjugate analysis of tumor infiltrating lymphocytes in human glioblastoma. Proc Am Assoc Cancer 300: 2380
Wrann M, Bodmer S, de Martin R, et al. (1987) T cell suppressor factor from human gliobastoma cells is a 12.5-kd protein closely related to transforming growth factor B. EMBO J 6: 1633–1636
Yagita M, Itoh K, Tsudo M, et al. (1989) Involvement of both Tac and non Tac inteleukin-2 binding peptides in the interleukin-2 dependent proliferation of human tumor infiltrating lymphocyte. Cancer Res 49: 1154–1159
Author information
Authors and Affiliations
Additional information
This work was supported by a grant from the Dunn Foundation, Houston, Texas
Rights and permissions
About this article
Cite this article
Grimm, E.A., Bruner, J.M., Carinhas, J. et al. Characterization of interleukin-2-initiated versus OKT3-initiated human tumor-infiltrating lymphocytes from glioblastoma multiforme: Growth characteristics, cytolytic activity, and cell phenotype. Cancer Immunol Immunother 32, 391–399 (1991). https://doi.org/10.1007/BF01741334
Issue Date:
DOI: https://doi.org/10.1007/BF01741334