Summary
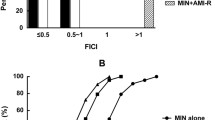
Thein vitro activity of clarithromycin alone and in combination with its primary human metabolite, 14-hydroxy-clarithromycin, was determined against 203 strains ofHaemophilus influenzae. Microdilution broth MICs and MBCs of both clarithromycin and 14-hydroxy-clarithromycin were determined. The clarithromycin MIC50 was 4 mg/l and the MIC90 was 8 mg/l. The hydroxy metabolite was 2–4-fold more active with an MIC50 and MIC90 of 2 mg/l. The MBCs were equal to the MICs. The microbicidal effect of combinations of clarithromycin and 14-hydroxy-clarithromycin was tested using a microdilution checkerboard technique and the fractional inhibitory index was calculated. The combination was additive in 92% and synergistic in 8% of all strains ofH. influenzae tested; no antagonism was found. The results were independent of the site of isolation of the strain or presence of β-lactamase. These findings suggest the potential clinical utility of clarithromycin for the treatment ofH. influenzae infections.
Zusammenfassung
DieIn-vitro-Aktivität von Clarithromycin allein und in Kombination mit seinem primären Metaboliten beim Menschen, 14-hydroxy-Clarithromycin, wurde bei 203 Stämmen vonHaemophilus influenzae geprüft. Für Clarithromycin wie seinen 14-hydroxy-Metaboliten wurden die MHK-und MBK- Werte mit der Mikrodilutions-Methode in Bouillon bestimmt. Die MHK50 von Clarithromycin betrug 4 mg/l, die MHK90 8% mg/l. Für den Hydroxy-Metaboliten ergab sich eine 2–4-fach höhere Aktivität mit MHK50- und MHK90-Werten von 2 mg/l. Die MBK-Werte waren mit den MHK-Werten identisch. Mit der Checkerboard-Methode wurde die mikrobizide Wirkung von Kombinationen aus Clarithromycin und 14-hydroxy-Clarithromycin bestimmt und der fraktionierte Hemmquotient berechnet. Für 92% aller Teststämme war die Wirkung der Kombination additiv, für acht synergistisch. Es fand sich kein Antagonismus. Die Ergebnisse waren unabhängig von der Isolations-Lokalisation oder der Anwesenheit von β-Laktamase. Diese Befunde lassen annehmen, daß Clarithromycin für die Behandlung vonH. influenzae-Infektionen geeignet ist.
Similar content being viewed by others
References
Hardy, D. J., Guay, D. R. P., Jones, R. N. Clarithromycin, a unique macrolide: a pharmacokinetic, microbiologic and clinical overview. Diagn. Microbiol. Infect. Dis. 15 (1992) 39–53.
Floyd-Reising, S., Hindler, J. A., Young, L. S. In vitro activity of A-56268 (TE-031), a new macrolide antibiotic, compared with that of eryhtromycin and other antimicrobial agents. Antimicrob. Agents Chemother. 31 (1987) 640–642.
Fernandes, P. B., Hardy, D., Bailer, R., McDonald, E., Pintar, J., Ramer, N., Swanson, R., Gade, E. Susceptibility testing of macrolide antibiotics againstHaemophilus influenzae and correlation ofin vitro results within vivo efficacy in a mouse septicemia model. Antimicrob. Agents Chemother. 31 (1987) 1243–1250.
Hardy, D. J., Swanson, R. N., Rode, R. A., Marsh, K., Shipkowitz, N. A., Clement, J. J. Enhancement of thein vitro andin vivo activities of clarithromycin againstHaemophilus influenzae by 14-hydroxy-clarithromycin, its major metabolite in humans. Antimicrob. Agents Chemother. 34 (1990) 1407–1413.
Dabernat, H., Delmas, C., Seguy, M., Fourtillan, J. B., Girault, Lareng M. B. The activity of clarithromycin and its 14-hydroxymetabolite againstHaemophilus influenzae, determined byin vitro and serum bactericidal tests. J. Antimicrob. Chemother. 27 (Suppl. A) (1991) 19–30.
Jorgensen, J. H., Maher, L. A., Howell, A. W. Activity of clarithromycin and its principal human metabolite againstHaemophilus influenzae. Antimicrob. Agents Chemother. 35 (1991) 1524–1526.
Tremblay, L. D., L'Ecuyer, J., Provencher, P., Bergeron, M. G., Canadian Study Group Susceptibility ofHaemophilus influenzae to antimicrobial agents used in Canada. Can. Med. Assoc. J. 143 (1990) 895–900.
Kilian, M. A rapid method for the differentiation ofHaemophilus influenzae strains. The porphyrin tests. Acta Pathol. Microbiol. Scand. (B). 82 (1974) 835–842.
Kilian, M. A taxonomic study of the genusHaemophilus with the proposal of new species. J. Gen. Microbiol. 93 (1976) 9–62.
O'Callaghan, C. H., Morris, A., Kirby, S. M., Sheigler, A. H. Novel method for detection of β-lactamase by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1 (1972) 283–288.
National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Second edition; Approved Standard. NCCLS Document M7-A2. Villanova, Pa.: NCCLS; 1990.
Jorgensen, J. H., Redding, J. S., Maher, L. A., Howell, A. W. Improved medium for antimicrobial susceptibility testing ofHaemophilus influenzae. J. Clin. Microbiol. 25 (1987) 2075–2113.
Eliopoulos, G. M., Moellering, R. C. Jr. Antimicrobial combinations. In:Lorian, V. (ed.): Antibiotics in laboratory medicine. Williams and Wilkins Co., Baltimore, Maryland, 1991, pp. 432–492.
Bergeron, M. G., Simard, P., Provencher, P. Influence of growth medium and supplement on growth ofHaemophilus influenzae and on antibacterial activity of several antibiotics. J. Clin. Microbiol. 25 (1987) 650–655.
Hanson, C. W., Bailer, R., Gade, E., Rode, R. A., Fernandes, P. B. Regression analysis, proposed interpretive zone size standards, and quality control guidelines for a new macrolide antimicrobial agent, A-56268 (TE-031). J. Clin. Microbiol. 25 (1987) 1079–1082.
Hoover, W. W., Barrett, M. S., Jones, R. N. Clarithromycinin vitro activity enhanced by its major metabolite, 14-hydroxy-clarithromycin. Diagn. Microbiol. Infect. Dis. 15 (1992) 259–266.
Wettengel, R.: Comparison of clarithromycin and cefaclor in the treatment of mild to moderate bronchitis. Abstracts of the 17th International Congress of Chemotherapy, Abstract No. 1823, Berlin, Germany, June 23–28, 1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bergeron, M.G., Bernier, M. & L'Ecuyer, J. In vitro activity of clarithromycin and its 14-hydroxy-metabolite against 203 strains ofHaemophilus influenzae . Infection 20, 164–167 (1992). https://doi.org/10.1007/BF01704612
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01704612