Summary
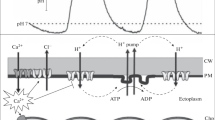
The present paper describes the construction and properties of a Pt/Ir-semi-microelectrode and its application as a redoxsensitive electrode in intact cells of the giant algaNitella. For compartmental analysis of the stationary redox-state voltage (ERED), a value reflecting the interaction of the dominant redox couples with a Pt/Ir-electrode, the redox-sensitive electrode was inserted into the vacuole of leaf cells or cytoplasm enriched fragments (CEF) fromNitella internodal cells. After correction for the membrane voltage, measured with a second, conventional voltage electrode, ERED values of+237±93mVand+419±51 mV with respect to a normal H+-electrode were obtained for cytoplasm and vacuole, respectively. The redox-state of the cell culture medium was+604 mV. The steady state ERED in the cytoplasm can be perturbed by experimental treatments: indirect acidification of the cytoplasm by an external pH jump from 7.5 to 5.8 and direct acidification, by acid loading with 5 mM butyrate, both resulted in a positive shift of ERED, i.e., to an increase in cytoplasmic oxidation. At the same time the membrane depolarized electrically following the external pH jump, but hyperpolarized in response to acid loading. The data demonstrate the direct dependence of cytoplasmic redox state on intracellular pH, probably due to enhanced oxidation of protonated redox couples favoured by mass action. The electrical membrane voltage changes were not correlated with the shift in cytoplasmic ERED. This demonstrated that redox energy does not determine the electrical membrane voltage. Cytoplasmic ERED was also affected by photosynthesis. When CEFs were transferred from light to dark, or exposed to 10μM 3-(3,4-dichlorophenyl)-1,l-dimethylurea (DCMU), ERED shifted negatively (more reduced) by 6.4±4.5mV or 4.2±2mV, respectively. These data compare favourably with biochemical estimates of cytoplasmic pyridin nucleotides which also show an increase in cytoplasmic reduction in the dark. Therefore, it is unlikely that diffusable reducing equivalents are supplied to the cytoplasm from photosynthetically-active chloroplasts to act as secondary messengers.
Similar content being viewed by others
Abbreviations
- EM :
-
transmembrane voltage
- ERED :
-
redox-state voltage
- E0 :
-
midpoint-redox-voltage
- APW:
-
artificial pond water
- CEF:
-
cytoplasm enriched fragment
References
Beilby MJ (1989) Electrophysiology of giant algal cells. Methods Enzymol 174: 403–443
—, Shepherd VA (1989) Cytoplasm-enriched fragments ofChara: structure and electrophysiology. Protoplasma 48: 150–163
Bertl A, Slayman CL (1990) Cation-selective channels in the vacuolar membrane ofSaccharomyces: dependence on calcium, redoxstate, and voltage. Proc Natl Acad Sci USA 87: 7824–7828
Brügger M, Fischer-Schliebs E, Lüttge U (1992) Einfluß verschiedener SH-Reagenzien auf die Plasmalemma-H+-ATPase aus Maiswurzeln. In: Haschke H-P, Schnarrenberg C (eds) Botanikertagung 1992, Berlin. Akademie-Verlag, Berlin, p 350
Cater DB, Philips AF, Silver IA (1957) Apparatus and techniques for measurements of oxidation-reduction potentials, pH and oxygen tension in vivo. Proc R Soc Lond [Biol] 146: 289–297
Coleman HA (1986) Chloride currents inChara —a patch clamp study. J Membrane Biol 93: 41–49
Elzenga JTM, Staal M, Prins HBA (1989) ATPase activity of isolated plasmalemma vesicles of leaves ofElodea are affected by thiole reagents and NADH/NAD ratio. Physiol Plant 76: 379–385
Heineke D, Riens B, Grosse H, Hoferichter P, Peter U, Flügge U-I, Heldt HW (1991) Redox transfer across the inner chloroplast envelope membrane. Plant Physiol 95: 1131–1137
Kochian LV, Lucas WJ (1982) Potassium transport in corn roots. I. Resolution of kinetics into a saturable and linear component. Plant Physiol 70: 1723–1731
Lichtner F, Lucas WJ, Spanswick RM (1981) Effect of sulfhydryl reagents on the biophysical properties of the plasmalemma ofChora corallina. Plant Physiol 68: 899–904
Lucas WJ, Alexander JM (1980) Sulfhydryl group involvement in plasmalemma transport of HCO3 − and OH− inChara corallina. Plant Physiol 65: 274–280
Mahler HR, Cordes EH (1967) Biological chemistry. Harper®ow, New York
Malbon CC, George ST, Moxham CP (1987) Intramolecular disulfide bridges: avenues to receptor activation? Trends Biochem Sci 12: 172–175
Mimura T, Kirino Y (1984) Changes in cytoplasmic pH measured by31P-NMR in cells ofNitettopsis obtusa. Plant Cell Physiol 25: 813–820
Møller IM, Lin W (1986) Membrane-bound NAD(P)H dehydrogenase in higher plant cells. Annu Rev Plant Physiol 37: 309–334
Okihara K, Kiyosawa K (1988) Ion composition of theChara internode. Plant Cell Physiol 29: 21–25
Puppi A, Dely M, Prager P (1976) Comparison of the redox-states of different tissues and types of acetylcholine effect. Acta Biochim Biophys Acad Sci Hung 11: 63–73
— — — (1980) Redox agents affecting drug actions. Gen Pharmacol 1: 409–418
Reid RJ, Smith FA (1988) Measurement of the cytoplasmic pH ofChara corallina using double-barreled pH microelectrodes. J Exp Bot 39: 1421–1432
Roth Z, Chayen N, Dickstein S (1983) The involvement of intra-cellular redox-state and pH in the metabolic control of stimulus-response coupling. Int Rev Cytol 85: 39–61
Rubinstein B, Stern AI (1991) The role of the plasma membrane redox activity in light effects in plants. J Bioenerg Biomembr 23: 393–408
Sanders D (1990) Kinetic modeling of plant and fungal transport systems. Annu Rev Plant Physiol Plant Mol Biol 41: 77–107
—, Miller AJ (1986) Measurements of cytoplasmic calcium activity with ion-selective microelectrodes. In: Trewavas AJ (ed) Molecular and cellular aspects of calcium in plant development. Plenum, London, pp 149–156
—, Hansen U-P, Slayman CL (1981) Role of the plasma membrane proton pump in pH regulation in non-animal cells. Proc Natl Acad Sci USA 78: 5903–5907
Serrano EE, Zeiger E, Hagiwara S (1988) Red light stimulates an electrogenic proton pump inVicia guard cell protoplasts. Proc Natl Acad Sci USA 85: 436–440
Spalding EP, Slayman CI, Goldsmith MHM, Gradmann D, Berti A (1992) Ion channels inArabidopsis plasma membrane. Transport characteristics and involvement in light induced voltage changes. Plant Physiol 99: 96–102
Takeshige K, Mitsumori F, Tazawa M, Mimura T (1992) Role of cytoplasmic inorganic phosphate in light-induced activation of H+-pumps in the plasma membrane and tonoplast ofChara corallina. Planta 186: 466–472
Tazawa M, Kishimoto U, Kikuyama M (1974) Potassium, sodium, and chloride in the protoplasm of Characeae. Plant Cell Physiol 15: 103–110
Thaler M, Simonis W, Schönknecht G (1992) Light-dependent changes of the cytoplasmic H+ and Cl−activity in the green algaEremosphaera viridis. Plant Physiol 99: 103–110
Thiel G, MacRobbie EAC, Hanke DE (1990) Raising the intra-cellular level of inositol 1,4,5-trisphosphate changes plasma membrane ion transport in characean algae. EMBO J 9: 1737–1741
Walz D (1979) Thermodynamics of oxidation-reduction reactions and its application to bioenergetics. Biochim Biophys Acta 505: 279–353
Winter U, Kirts GO (1990) Salinity response of a freshwater charophyte,Chara vulgaris. Plant Cell Environ 13: 123–134
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thiel, G. Redox-state of intactNitella cells: dependency on intracellular pH and photosynthesis. Protoplasma 179, 26–33 (1994). https://doi.org/10.1007/BF01360734
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01360734