Summary
-
1.
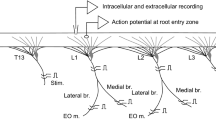
The largest crayfish tonic flexor motoneuron, f6, exhibits a patterned discharge in the form of repetitive bursts. The mechanism of burst production was examined using intracellular and extracellular recording and stimulation of identified tonic flexor motoneurons in the third or fourth abdominal ganglia.
-
2.
Depolarizing currents (over a restricted range) injected into the somata or neuropilar processes of f6 result in the clustered discharge characteristic of ‘command’ or sensory induced activation (Fig. 4).
-
3.
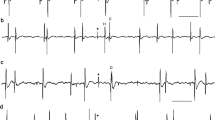
Both driven and spontaneous bursts in the motoneuron are associated with unpatterned synaptic activity (Fig. 5, 6, 7).
-
4.
The frequency-current (f-I) relationship reveals no increase in slope or average sensitivity over the range of injected currents which give rise to bursting (Fig. 5).
-
5.
Impulses generated during a burst, as seen in neuropilar impalements, are associated with a depolarizing afterpotential (DAP). The DAP appears to arise from a passive invasion of dendritic membrane by the preceding impulse (Figs. 7, 8).
-
6.
The time course of the DAP is closely paralleled by an increase in excitability of the spike initiation zone following a singlet action potential. The excitability cycle following a rhythmic impulse is a good predictor of the ‘preferred’ intradoublet (intraburst) intervals (Fig. 9A-C).
-
7.
Each burst terminates with a prominent postburst hyperpolarization (PBH) (Fig. 10).
It is concluded that burst formation is endogenous to the motoneuron andnot strongly dependent on patterned presynaptic input.
Similar content being viewed by others
Abbreviations
- DAP :
-
depolarizing afterpotential
- PBH :
-
postburst hyperpolarization
- ISI :
-
interspike interval
- f-I :
-
frequency-current
- EPSP :
-
excitatory postsynaptic potential
- IPSP :
-
inhibitory postsynaptic potential
References
Anderson P, Eccles JC, Sears TA (1964) The ventro-basal complex of the thalamus: Types of cells, their responses and their functional organization. J Physiol 174:370–399
Arvanitaki A, Chalazonitis N (1967) Electrical properties and temporal organization in oscillatory neurons (Aplysia). In: Salanki J (ed) Neurobiology of invertebrates. Akademiai Kiado, Budapest. Plenum Press, New York, 1968, pp 169–199
Atwood HL (1973) An attempt to account for the diversity of crustacean muscles. Am Zool 13:357–378
Bullock TH, Terzuolo CA (1957) Diverse forms of activity in the somata of spontaneous and integrating ganglion cells. J Physiol 138:341–364
Calvin WH (1973) A third mode of repetitive firing: Self-regenerative firing due to large delayed depolarizations. In: Stein RB, Pearson KG, Smith RS, Redford JB (eds) Control of posture and locomotion. Plenum Press, New York, pp 173–178
Calvin WH (1974) Three modes of repetitive firing and the role of threshold time course between spikes. Brain Res 69:341–346
Calvin WH (1975) Generation of spike trains in CNS neurons. Brain Res. 84:1–22
Calvin WH, Hartline DK (1977) Retrograde invasion of lobster stretch receptor somata in control of firing rate and extra spike patterning. J Neurophysiol 40:106–118
Calvin WH, Schwindt PC (1972) Steps in production of motoneuron spikes during rhythmic firing. J Neurophysiol 35:297–310
Calvin WH, Sypert GW (1976) Fast and slow pyramidal tract neurons: An intracellular analysis of their contrasting repetitive firing properties in the cat. J Neurophysiol 39:420–434
Combs AL, Mulligan BE, Taylor RC (1979) Resistance reflexes and patterned discharge in motor neurons of the crayfish propusdactylus joint. Comp Biochem Physiol 64:49–58
Connor JA (1975) Neural repetitive firing: A comparative study of membrane properties of crustacean walking leg axons. J Neurophysiol 38:922–932
Davis WJ (1971) Functional significance of motoneuron size and soma position in swimmeret system of the lobster. J Neurophysiol 34:274–288
Evoy WH, Kennedy D (1967) The central organization underlying control of antagonistic muscles in the crayfish. I. Types of command fibers. J Exp Zool 165:223–237
Evoy WH, Kennedy D, Wilson DM (1967) Discharge patterns of neurones supplying tonic abdominal flexor muscles in the crayfish. J Exp Biol 46:393–411
Eyzaguirre C, Kuffler SW (1955) Further study of soma, dendrite, and axon excitation in single neurons. J Gen Physiol 39:121–154
Fields HL (1966) Proprioceptive control of posture in the crayfish abdomen. J Exp Biol 44:455–468
Getting PA, Willows AOD (1974) Modification of neuron properties by electrotonic synapses. II. Burst formation by electrotonic synapses. J Neurophysiol 37:848–868
Gillary HL, Kennedy D (1969a) Pattern generation in a crustacean motoneuron. J Neurophysiol 32:595–606
Gillary HL, Kennedy D (1969b) Neuromuscular effects of impulse pattern in a crustacean motoneuron. J Neurophysiol 32:607–6612
Glantz RM (1978a) Crayfish antennal neuropil. I. Reciprocal synaptic interactions and input-output characteristics of first-order interneurons. J Neurophysiol 41:1297–1313
Glantz RM (1978b) Crayfish antennal neuropil. II. Periodic bursting elicited by sensory stimulation and extrinsic current in interneurons. J Neurophysiol 41:1314–1327
Grampp W (1966a) Multiple-spike discharge evoking after-depolarizations in the slowly adapting stretch receptor neuron of the lobster. I. The labile and fast after-depolarization. Acta Physiol Scand 67:100–115
Grampp W (1966b) Multiple-spike discharge evoking after-depolarizations in the slowly adapting stretch receptor neuron of the lobster. II. The slow after-depolarization. Acta Physiol Scand 67:116–126
Granit R, Kernell, D, Smith RS (1963) Delayed depolarization and the repetitive response to intracellular stimulation of mammalian motoneurons. J Physiol 168:890–910
Harreveld A Van (1936) A physiological solution for fresh water Crustacea. Proc Soc Exp Biol Med 34:428–432
Hartline DK (1979) Pattern generation in the lobster (Panulirus) stomatogastric ganglion. II. Pyloric network simulation. Biol Cybern 33:223–236
Hartline DK, Gassie DV Jr (1979) Pattern generation in the lobster (Panulirus) stomatogastric ganglion. I. Pyloric neuron kinetics and synaptic interactions. Biol Cybern 33:209–222
Hermann A (1979) Generation of a fixed motor pattern. II. Electrical properties and synaptic characteristics of pyloric neurons in the stomatogastric ganglion of the crabCancer pagurus. J Comp Physiol 130:229–239
Hodgkin AL (1948) The local electrical changes associated with repetitive action in a non-medullated axon. J Physiol 107:165–181
Ito M, Oshima T (1962) Temporal summation of afterhyperpolarization following a motoneurone spike. Nature 195:910–911
Jansen JKS, Nicholls JG (1973) Conductance changes, an electrogenic pump, and the hyperpolarization of leech neurones following impulses. J Physiol 229:635–655
Junge D, Stephens CL (1973) Cycle variation of potassium conductance in a burst-generating neurone inAplysia. J Physiol 235:155–181
Kandel ER (1976) Cellular basis of behavior. Freeman, San Francisco
Kandel ER, Spencer WA (1961) Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol 24:243–259
Kater SB, Kaneko CRS (1972) An endogenously bursting neuron in the gastropod mollusc,Helisoma trivolvis. J Comp Physiol 79:1–14
Kennedy D, Davis WJ (1977) Organization of invertebrate motor systems. In: Brookhart JM, Montcastle VV, Kandel ER (eds) Handbook of physiology, sect 1, vol. 1. American Physiological Society, Bethesda, pp 1023–1087
Kennedy D, Takeda K (1965b) Reflex control of abdominal flexor muscles in the crayfish. II. The tonic system. J Exp Biol 43:229–246
Kennedy D, Evoy WH, Fields HL (1966) The unit basis of some crustacean reflexes. Symp Soc Exp Biol 20:75–109
Kirk MD, Glantz RM (1980) Investigation of burst formation in a crayfish abdominal postural motoneuron. Neurosci Abstr 6:372
Koester J, Mayeri E, Liefeswar G, Kandel ER (1974) Neural control of circulation inAplysia. II. Interneurons. J Neurophysiol 37(3):476–496
Kuwada JY, Hagiwara G, Wine JJ (1980) Postsynaptic inhibition of crayfish tonic flexor motor neurons by escape commands. J Exp Biol 84:343–348
Larimer JL, Eggleston AC (1971) Motor programs for abdominal positioning in crayfish. Z Vergl Physiol 74:388–402
Maynard DM (1966) Integration in crustacean ganglia. Symp Soc Exp Biol 20:111–149
Mellon De F, Kaars C (1974) Role of regional cellular geometry in conduction of excitation along a sensory neuron. J Neurophysiol 37:1228–1238
Merickel M, Gray R (1980) Investigation of burst generation by the electrically coupled cyberchron network in the snailHelisoma using a single-electrode voltage clamp. J Neurobiol 11:73–102
Murphey RK (1973) Characterization of an insect neuron which cannot be visualized in situ. In: Kater SB, Nicholson C (eds) Intracellular staining in neurobiology. Springer, Berlin Heidelberg New York, pp 135–150
Nelson PG, Burke RE (1967) Delayed depolarization in cat spinal motoneurons. Exp Neurol 17:16–26
Ogden TE, Citron MC, Pierantoni R (1978) The jet stream microbeveler: An inexpensive way to bevel ultrafine glass micropipettes. Science 201:469–470
Parnas I, Atwood HL (1966) Phasic and tonic neuromuscular systems in the abdominal extensor muscles of the crayfish and rock lobster. Comp Biochem Physiol 18:701–723
Perkel DH, Mulloney B (1974) Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science 185:181–182
Perkel DH, Gerstein GL, Moore GP (1967) Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J 7:391–418
Sandeman DC (1969) Integrative properties of a reflex motoneuron in the brain of the crabCarcinus maenas. Z Vergl Physiol 64:450–464
Selverston AI, Russell DF, Miller JP, King DC (1976) The stomatogastric nervous system: Structure and function of a small neural network. Progr Neurobiol 6:1–75
Siegler MVS, Mpitsos GJ, Davis WJ (1974) Motor organization and generation of rhythmic feeding output in buccal ganglion ofPleurobranchia. J Neurophysiol 37:1173–1196
Sokolove PG, Cooke IM (1971) Inhibition of impulse activity in a sensory neuron by an electrogenic pump. J Gen Physiol 57:125–163
Sokolove PG, Tatton WG (1975) Analysis of postural motoneuron activity in crayfish abdomen. I. Coordination by premotoneuron connections. J Neurophysiol 38:313–331
Stent GS, Thompson WJ, Calabrese RL (1979) Neural control of heartbeat in the leech and in some other invertebrates. Physiol Rev 59:101–136
Stewart WW (1978) Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell 14:741–159
Strumwasser F (1967) Types of information stored in single neurons. In: Wiersma CAG (ed) Invertebrate nervous systems. Univ. of Chicago Press, Chicago, pp 291–319
Takeda K, Kennedy D (1964) Soma potentials and modes of activation of crayfish motoneurons. J Cell Comp Physiol 64: 165–182
Tatton WG, Sokolove PG (1975) Analysis of postural motoneuron activity in crayfish abdomen. II. Coordination by excitatory and inhibitory connections between motoneurons. J Neurophysiol 38:332–346
Tazaki K, Cooke IM (1979) Isolation and characterization of slow depolarizing responses of cardiac ganglion neurons in the crab,Portunus sanguinolentus. J Neurophysiol 42:1000–1021
Thompson CS, Page CH (1979) Control of superficial flexor efferents by abdominal connective fibers in the lobster. Neurosci Abstr 5:263
Thompson SH, Smith SJ (1976) Depolarizing afterpotentials and burst production in molluscan pacemaker neurons. J Neurophysiol 39:153–161
Tomita T, Wright EB (1965) A study of the crustacean axon repetitive response. I. The effect of membrane potential and resistance. J Cell Comp Physiol 65:195–210
Vélez SJ, Wyman FJ (1978a) Synaptic connectivity in a crayfish neuromuscular system. I. Gradient of innervation and synaptic strength. J. Neurophysiol 41:75–84
Vélez SJ, Wyman FJ (1978b) Synaptic connectivity in a crayfish neuromuscular system. II. Nerve-muscle matching and nerve branching patterns. J Neurophysiol 41:85–96
Watanabe A, Obara S, Akiyama T (1967) Pacemaker potentials for the periodic burst discharge in the heart ganglion of a stomatopod,Squitta oratoria. J. Gen Physiol 50:839–863
Wiens TJ (1976) Electrical and structural properties of crayfish claw motoneurons in an isolated claw-ganglion preparation. J Comp Physiol 112:213–233
Wilson DM (1964) Relative refractoriness and patterned discharge of locust flight motor neurons. J Exp Biol 41:191–205
Wilson DM, Davis WJ (1965) Nerve impulse patterns and reflex control in the motor system of the crayfish claw. J Exp Biol 43:193–210
Wine JJ, Mittenthal JE, Kennedy D (1974) The structure of tonic flexor motoneurons in crayfish abdominal ganglia. J Comp Physiol 93:315–335
Zollman JR, Gainer H (1971) Electrophysiological properties of nerve cell bodies in the sixth abdominal ganglion of the Maine lobster,Homarus americanus. Comp Biochem Physiol 38A: 407–433
Author information
Authors and Affiliations
Additional information
The authors would like to thank Mr. Bruce Hughes and Ms. Mary LeBus for their expert assistance with the figures. Thanks to Mr. Brian Waldrop and Dr. Harvey Nudelman for their helpful discussions and critical reading of the manuscript, and to Dr. Terry Viancour for suggesting the experiments illustrated in Fig. 10 A, B. Special appreciation for many helpful discussions and technical assistance during the early phases of this work goes to Ms. Mary LeBus. Supported by N.S.F. Grant No. BNS-7910335.
Rights and permissions
About this article
Cite this article
Kirk, M.D., Glantz, R.M. Impulse pattern generation in a crayfish abdominal postural motoneuron. J. Comp. Physiol. 141, 183–196 (1981). https://doi.org/10.1007/BF01342665
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01342665