Summary
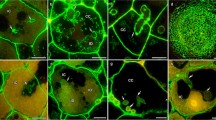
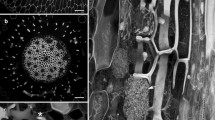
Early responses of legume roots toRhizobium inoculation include new cell wall synthesis and induction of some putative wall protein genes. Although the predicted amino acid sequences of several early nodulins indicate that they encode proline-rich proteins (PRPs), the proteins have been neither isolated nor has their presence been demonstrated in cell walls. We have used polyclonal antibodies against PRP2 from soybean to identify and localize proline-rich proteins in pea nodules. On immunoblots, several PRPs were detected, ranging from less than 20 kDa to 110 kDa. Immunocytochemistry revealed that tissues of the vascular cylinder contained abundant PRPs, particularly in the secondary cell walls of xylem elements and phloem fibers. PRPs were also found within the primary wall of the nodule endodermis and within Casparian strips of the vascular endodermis. Of symbiotic importance, PRPs were a prominent component of the infection thread matrix in newly infected root cells and in nodules. PRPs were also secreted by cells in the uninfected nodule parenchyma, where they were found occluding intercellular spaces outside the middle lamella. Despite structural conservation among members of this class of cell wall proteins, PRPs were targeted to distinct layers of the extracellular matrix dependent upon cell type, and may thus play separate roles in the biology of plant cells. The putative functions and the potential for interactions between PRPs and other wall polymers are discussed.
Similar content being viewed by others
Abbreviations
- DTT:
-
dithiothreitol
- EDTA:
-
ethylenediamine tetraacetate
- GRP:
-
glycine-rich protein
- PCR:
-
polymerase chain reaction
- PGA:
-
polygalacturonic acid
- PMSF:
-
phenylmethylsulfonyl fluoride
- PRP:
-
proline-rich protein
- SDS-PAGE:
-
sodium dodecylsulfate-polyacrylamide gel electrophoresis
- Tris:
-
tris(hydroxylmethyl) aminomethane
- Tween:
-
20 polyoxyethylene sorbitan monolaurate
References
Bonnett HT Jr (1968) The root endodermis: fine structure and function. J Cell Biol 37: 199–205
Bradley DJ, Kjellbom P, Lamb CJ (1992) Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70: 21–30
—, Wood EA, Larkins AP, Galfre G, Butcher GW, Brewin NJ (1988) Isolation of monoclonal antibodies reacting with peribacteroid membranes and other components of pea root nodules containingRhizobium leguminosarum. Planta 173: 149–160
Brewin NJ (1991) Development of the legume root nodule. Annu Rev Cell Biol 7: 191–226
—, Wood EA, Young JPW (1983) Contribution of the symbiotic plasmid to the competitiveness ofRhizobium leguminosarum. J Gen Microbiol 129: 2973–2977
Callaham DA, Torrey JG (1981) The structural basis for infection of root hairs ofTrifolium repens byRhizobium Can J Bot 59: 1647–1664
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30
Datta K, Schmidt A, Marcus A (1989) Characterization of two soybean repetitive proline-rich proteins and a cognate cDNA from germinated axes. Plant Cell 1: 945–952
Espelie K, Kolattukudy PE (1985) Purification and characterization of an abscisic acid-inducible anionic peroxidase associated with suberization in potato (Solanum tuberosum). Arch Biochem Biophys 240: 539–545
Esau K (1977) Anatomy of seed plants, 2nd edn. Wiley, New York
Franssen HJ, Nap J-P, Bisseling T (1992) Nodulins in root nodule development. In: Stacey G, Evans HJ, Burris RH (eds) Biological nitrogen fixation. Chapman and Hall, New York, pp 598–624
Fry SC (1986) Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol 37: 165–186
Glenn AR, Brewin NJ (1981) Succinate-resistant mutants ofRhizobium leguminosarum. J Gen Microbiol 126: 237–241
Govers F, Harmsen H, Heidstra R, Michielsen P, Prins M, van Kammen A, Bisseling T (1991) Characterization of the peaENOD 12B gene and expression analyses of the twoENOD 12 genes in nodule, stem and flower tissue. Mol Gen Genet 228: 160–166
Gunning BES, Steer MW (1986) Plant cell biology: an ultrastructural approach. MW Steer, Dublin
Hirsch AM (1992) Developmental biology of legume nodulation. New Phytol 122: 211–237
James EK, Sprent JI, Minchin FR, Brewin NJ (1991) Intercellular location of glycoprotein in soybean nodules: effect of altered rhizosphere oxygen concentration. Plant Cell Environ 14: 467–476
Keller B (1993) Structural cell wall proteins. Plant Physiol 101: 1127–1130
—, Templeton MD, Lamb CJ (1989) Specific localization of a plant cell wall glycine-rich protein in protoxylem cells of the vascular system. Proc Natl Acad Sci USA 86: 1529–1533
Kieliszewski M, Lamport DTA (1994) Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J 5: 157–172
Kijne JW (1992) TheRhizobium infection process. In: Stacey G, Evans HJ, Burris RH (eds) Biological nitrogen fixation. Chapman and Hall, New York, pp 349–398
Knox JP, Linstead PJ, King J, Cooper C, Roberts K (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512–521
Kolattukudy PE (1984) Biochemistry and function of cutin and suberin. Can J Bot 62: 2918–2933
Löbler M, Hirsch AM (1993) A gene that encodes a proline-rich nodulin with limited homology toPsENOD 12 is expressed in the invasion zone ofRhizobium meliloti-induced alfalfa root nodules. Plant Physiol 103: 21–30
Marcus A, Greenberg J, Averyhart-Fullard V (1991) Repetitive proline-rich proteins in the extracellular matrix of the plant cell. Physiol Plant 81: 273–279
Newcomb W (1976) A correlated light and electron microscopic study of symbiotic growth and differentiation inPisum sativum root nodules. Can J Bot 54: 2163–2186
Parsons R, Day DA (1990) Mechanism of soybean nodule adaptation to different oxygen pressures. Plant Cell Environ 13: 501–512
Pate JS, Gunning BES, Briarty LG (1969) Ultrastructure and functioning of the transport system of the leguminous root nodule. Planta 85: 11–34
Pichon M, Journet E, Dedieu A, de Billy F, Truchet G, Barker DG (1992)Rhizobium meliloti elicits transient expression of the early nodulin geneENOD 12 in the differentiating root epidermis of transgenic alfalfa. Plant Cell 4: 1199–1211
Rae AL, Perotto S, Knox JP, Kannenberg EL, Brewin NJ (1991) Expression of extracellular glycoproteins in the uninfected cells of developing pea nodule tissue. Mol Plant Microbe Interact 4: 563–570
—, Bonfante-Fasolo P, Brewin NJ (1992) Structure and growth of infection threads in the legume symbiosis withRhizobium leguminosarum. Plant J 2: 385–395
Ryser U, Keller B (1992) Ultrastructural localization of a bean glycine-rich protein in unlignified primary wall of protoxylem cells. Plant Cell 4: 773–783
Salzwedel JL, Dazzo FB (1993) pSymnod genes influence on elicitation of peroxidase activity from white clover and pea roots by rhizobia and their cell-free supernatants. Mol Plant Microbe Interact 6: 127–134
Scheres B, van de Wiel C, Zalensky A, Horvath B, Spaink H, van Eck H, Zwartkruis F, Wolters A-M, Gloudemans T, van Kammen A, Bisseling T (1990 a) The ENOD 12 gene product is involved in the infection process during the pea-Rhizobium interaction. Cell 60: 281–294
—, van Engelen F, van der Knaap E, van de Wiel C, van Kammen A, Bisseling T (1990 b) Sequential induction of nodulin gene expression in the developing pea nodule. Plant Cell 2: 687–700
Sherrier DJ, VandenBosch KA (1994) Secretion of cell wall polysaccharides inVicia root hairs. Plant J 5: 185–196
Showalter AM (1993) Structure and function of plant cell wall proteins. Plant Cell 5: 9–23
Sindhu SS, Brewin NJ, Kannenberg EL (1990) Immunocytochemical analysis of lipopolysaccharides from free-living and endosymbiotic forms ofRhizobium leguminosarum. J Bacteriol 172: 1804–1813
Tjepkema TJ, Yocum CS (1974) Measurement of oxygen partial pressure within soybean nodules by oxygen microelectrode. Planta 119: 351–360
Turgeon BG, Bauer WD (1985) Ultrastructure of infection-thread development during the infection of soybean byRhizobium japonicum. Planta 163: 328–349
VandenBosch KA (1991) Immunogold labelling. In: Hall JL, Hawes C (eds) Electron microscopy of plant cells. Academic Press, London, pp 181–218
—, Bradley DJ, Knox JP, Perotto S, Butcher GW, Brewin NJ (1989) Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J 8: 335–342
van de Wiel C, Scheres B, Franssen H, van Lierop M-J, van Lammeren A, van Kammen A, Bisseling T (1990) The early nodulin transcript ENOD 2 is located in the nodule parenchyma (inner cortex) of pea and soybean root nodules. EMBO J 9: 1–7
Witty JF, Minchin FR, Skot L, Sheehy JE (1986) Nitrogen fixation and oxygen in legume root nodules. Oxford Surv Plant Mol Cell Biol 3: 275–314
Wyatt RE, Nagao RT, Key JL (1992) Patterns of soybean proline-rich protein gene expression. Plant Cell 4: 99–110
Ye Z-H, Varner JE (1991) Tissue-specific expression of cell wall proteins in developing soybean tissues. Plant Cell 3: 23–37
—, Song Y-R, Marcus A, Varner JE (1991) Comparative localization of three classes of cell wall proteins. Plant J 1: 175–183
Zhang GF, Staehelin LA (1992) Functional compartmentation of the Golgi apparatus of plant cells. Immunocytochemical analysis of high pressure frozen and freeze-substituted sycamore maple suspension culture cells. Plant Physiol 99: 1070–1083
Author information
Authors and Affiliations
Additional information
Dedicated to the memory of Professor John G. Torrey
Rights and permissions
About this article
Cite this article
Sherrier, D.J., VandenBosch, K.A. Localization of repetitive proline-rich proteins in the extracellular matrix of pea root nodules. Protoplasma 183, 148–161 (1994). https://doi.org/10.1007/BF01276823
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01276823