Abstract
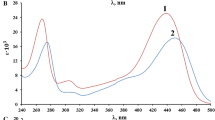
The oxidative coupling reactions of unsubstituted phenol, 4-cresol, 2,6-xylenol and another fourteen substituted phenols with MBTH in alkaline medium containing potassium hexacyanoferrate (III) as the oxidant were examined by spectrophotometry. The maximum yield and stability of coloured species was attained at optimum pH 9.0±0.5. Stoichiometry of the reactions involving a four-electron transfer was confirmed. The coloured products were stable in an aqueous alkaline medium containing 50% (V/V) of a water-miscible organic solvent and they could be quantitatively extracted into chloroform. Absorption maxima λmax of the reaction products ranged between 495 nm and 610 nm depending on the phenol structure and their molar absorptivities fell within the range 2,500–47,000L mol−1 cm−1 in aq. 50% ethanol. The calibration graphs were rectilinear for 2 to 20 μM phenol or 2,6-xylenol (r = 0.9994–0.9997; n = 6) and the RSD values were ≈1.8% (n=10) when determining 10 μmol L−1 of the analytes. The reactivity of the phenols with MBTH (and hence the yield of the coloured species in alkaline medium) depending on the analyte structure decreased in the order: 2,6-dialkylphenols or 4-halogenated 2,6-dialkylphenols>2-alkyl, 2-alkoxy or 2-arylphenols and l-naphthol>unsubstituted phenol or 2-naphthol≅2-halogenated phenols>4-alkylphenols and 4-halogenated phenols>2-nitrophenol>2,4-or 3,4-dialkylphenols. For some 4-halogenated phenols the elimination of halogen upon coupling with the MBTH was observed.
Similar content being viewed by others
References
S. Hunig, K. H. Fritsch,Liebigs Ann. Chem. 1957,609, 143.
S. Hunig, K. H. Fritsch,Liebigs Ann. Chem. 1957,609, 172.
E. Sawicki, T. R. Hauser, T. W. Stanley, W. Elbert,Anal. Chem. 1961,33, 93.
A. P. Altschuller, L. J. Leng,Anal. Chem. 1963,35, 1541.
A. Paz, O. O. Blumenfeld, M. Rojkind, E. Henson, C. Furfine, P. M. Gallop,Arch. Biochem. Biophys. 1965,109, 548 (Chem. Abstr. 1965,62, 120140e).
E. Kamata,Bull. Chem. Soc. Jap. 1965,38, 2005.
H. Verachtert, J. Frateur,J. Agr. Sci. 1966,14, 83.
M. Pays, P. Malangeau, R. Bourdon,Ann. Pharm. Fr. 1966,24, 763.
F. W. Neumann,Anal. Chem. 1969,41, 2077.
S. Hunig, H. Nother,Liebigs Ann. Chem. 1959,628, 69.
E. Sawicki, T. W. Stanley, T. R. Hauser, W. Elbert, J. Noe,Anal. Chem. 1961,33, 722.
M. Pays, R. Bourdon, M. Beljeam,Anal. Chim. Acta 1969,747, 101.
S. Hunig, H. Nother,Liebigs. Ann. Chem. 1959,628, 84.
E. Sawicki, T. R. Hauser, T. W. Stanley, W. Elbert, F. T. Fox,Anal. Chem. 1961,33, 1574.
K. Kalo, M. Umeda, S. Tsubota,Yakugaku Zasshi 1963,83, 1180 (Chem. Abstr. 1964,60, 11381).
E. Sawicki, T. W. Stanley, W. Elbert,Microchem. J. 1961,5, 225.
J. Bartos,Ann. Pharm. Fr. 1962,20, 650.
T. Naito, H. Nagano, T. Yasuji, T. Ishihara,Eisei Kagaku 1969,15, 244; (Chem. Abstr. 1970, 72, 86050).
E. Sawicki, R. Shumacker, C. R. Engel,Microchem. J 1967, 12, 377.
I. R. Riemschneider,Monatshefte 1958,89, 683.
E. Sawicki, C. R. Engel, M. Guyer,Anal. Chim. Acta 1967,39, 505.
S. Hunig, H. Balli,Liebigs Ann. Chem. 1959,628, 56.
M. Umeda,Yakugaku Zasshi 1963,83, 951.
H. Sakurai, M. Umeda,Yakugaku Zasshi 1963,83, 1000.
E. Kamata,Bull. Chem. Soc. Jpn. 1964,37, 1674.
E. Kamata,Nippon Kagaku Zasshi 1966,87, 380.
M. Pays, R. Bourdon,Ann. Pharm. Fr. 1968, 26, 681.
H. O. Friestad, D. E. Ott, F. Gunther,Anal. Chem. 1969,41, 1750.
P. Koppe, F. Dietz, J. Traud,Z. Anal. Chem. 1977,285, 1.
M. E. El-Komos,Arch. Pharm. 1982,10, 146.
S. Hunig,Angew. Chem. 1962,74, 818.
S. Hunig, H. Balli, K. H. Fritsch, H. Herrmann, G. Kobrich, H. Werner, E. Grigat, F. Muller, H. Nother, K. H. Oethe,Angew. Chem. 1958,70, 215.
S. Hunig, H. Balli, H. Nother, H. Geiger,Liebigs Ann. Chem. 1959,628, 75.
E. Sawicki, C. R. Engel,Analyst 1967,56, 7.
M. Pesez, J. Bartos,Ann. Pharm. Fr. 1964,22, 609.
J. Fog, E. Jellum,Nature 1962,195, 490.
J. Gasparic, D. Svobodová, M. Pospíšilová,Mikrochim. Acta [Wien] 1977,I, 241.
M. Pospíšilová, D. Svobodová, J. Gasparic, M. Machácek,Mikrochim. Acta [Wien] 1990,III, 117.
D. Svobodová, J. Gasparic,Mikrochim. Acta [Wien] 1975,II, 529.
H. Hosoda, W. Takasaki, T. Oe, R. Tsukamoto, T. Nambara,Chem. Pharm. Bull. 1986,34, 4177.
J. Riesenfeld, L. Roden,Anal. Biochem. 1990,188, 383.
J. Grewal, B. Mutus,Microchem. J. 1991,44, 276.
M. Del-Pilar-Castillo, J. Stenstrom, P. Ander,Anal. Biochem. 1994,218, 399.
G. Gori, P. Meneghetti, A. Sturaro, G. Parvoli, L. Doretti, G. B. Bartolucci,Chromatographia 1995,40, 336.
C. S. P. Sastry, A. M. Rao,Mikrochim. Acta 1989,I, 237.
M. B. Devani, S. S. Pandya, S. A. Shah,Indian. J. Pharm. Sci 1991,53, 96.
E. M. De-Almeida-Orsine, J. L. Seferin-Martins,Anal. Lett. 1993,26, 1933.
C. S. P. Sastry, K. R. Rao, D. S. Prasad,Indian Drugs 1995,32, 172.
M. PospísilováThesis, Charles University, Hradec Králové, 1986.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pospíšilová, M., Polášek, M. & Svobodová, D. Spectrophotometric study of reactions of substituted phenols with MBTH in alkaline medium: The effect of phenol structure on the formation of analytically useful coloured products. Mikrochim Acta 129, 201–208 (1998). https://doi.org/10.1007/BF01244742
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01244742