Summary
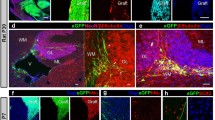
Previous attempts to generate myelin in the myelin-deficient rat spinal cord by transplanting mouse glia were not successful. In order to determine whether this result was due to graft rejection or to interspecies mismatch of cellular or molecular components at the axoglial junction, we have repeated the experiment in cyclosporine-treated rats. Our results show that in the immunosuppressed hosts, foetal glial xenografts form an abundance of myelin within the dorsal columns at or near the injection site about two weeks after the operation. In some cases, myelination extends virtually across the entire width of the dorsal columns. Ultrastructurally, the myelin sheaths are normal in all respects, including the presence of the ‘radial component’. The lateral edges of the myelin lamellae form typical paranodal axoglial junctions, some displaying periodic ‘transverse bands’. We infer that previous mouse to rat xenograft failures reflect host immune response rather than mismatch of heterologous junctional components. We also compared foetal, early post-natal and adult xenografts. Foetal donor cells, containing an abundance of precursors but virtually no mature oligodendrocytes, are more effective than neonatal donor cells in forming myelin, and after adult grafts, we found no myelin formation. Thus, in xenografts, as in allografts, foetal precursor cells are far more suitable than glia from mature donors in generating significant amounts of myelin.
Similar content being viewed by others
References
Archer, D. R., Huffman, R., Miletic, V. &Duncan, I. D. (1991) Myelination in the myelin deficient rat by transplanted canine glial cells.Society for Neuroscience Abstracts 17, 1149.
Blakemore, W. F. (1974) Remyelination of the superior cerebellar peduncle in old mice following demyelination induced by cuprizone.Journal of the Neurological Sciences 22, 121–6.
Blakemore, W. F. &Franklin, R. J. M. (1991) Transplantation of glial cells into the CNS.Trends in Neuroscience 14, 323–7.
Bunge, M. P., Bunge, R. B. &Ris, H. (1961) Ultrastructural study of remyelination in an experimental lesion of cat spinal cord.Journal of Biophysical and Biochemical Cytology 7, 685–96.
Crang, A. J. &Blakemore, W. F. (1991) Remyelination of demyelinated rat axons by transplantation of mouse oligodendrocytes.Glia 4, 305–13.
Dubois-Dalq, M. &Armstrong, R. (1990) The cellular and molecular events of central nervous system remyelination.BioEssays 12, 569–76.
Gumpel, M., Lachapelle, F., Gansmuller, A., Baulac, M., Baron Van Evercooren, A. &Baumann, N. (1987) Transplantation of human embryonic oligodendrocytes into shiverer brain.Annals of the New York Academy of Sciences 495, 71–85.
Hasegawa, M. &Rosenbluth, J. (1991) Transplantation of labeled fetal spinal cord fragments into juvenile myelin-deficient rat spinal cord.Anatomical Record 229, 138–43.
Hirano, A. &Dembitzer, H. M. (1982) Further studies on the transverse bands.Journal of Neurocytology 11, 851–6.
Hirano, A., Levine, S. &Zimmerman, H. M. (1968) Remyelination in the central nervous system after cyanide intoxication.Journal of Neuropathology and Experimental Neurology 27, 234–45.
Johnson, E. S. &Ludwin, S. K. (1981) The demonstration of recurrent demyelination and remyelination of axons in the central nervous system.Acta Neuropathologica 53, 93–8.
Koeppen, A. (1992) Pelizaeus-Merzbacher disease: X-linked proteolipid deficiency in the human central nervous system. InMyelin: Biology and Chemistry (edited byMartenson, R. E.) pp. 703–21. Boca Raton: CRC Press.
Kosaras, B. &Kirschner, D. A. (1990) Fine structure and supramolecular organization of the radial component of CNS myelin.Annals of the New York Academy of Sciences 605, 430–4.
Lampert, P. W. (1965) Demyelination and remyelination in experimental allergic encephalomyelitis. Further electron microscopic observations.Journal of Neuropathology and Experimental Neurology 24, 371–85.
Lawrence, J. M., Morris, R. J., Wilson, D. J. &Raisman, G. (1990) Mechanisms of allograft rejection in the rat brain.Neuroscience 37, 431–62.
Livingston, R. B., Pfenninger, K., Moor, H. &Akert, K. (1973) Specialized paranodal and interparanodal glialaxonal junctions in the peripheral and central nervous system: a freeze-etching study.Brain Research 58, 1–24.
Lubetzki, C., Gansmuller, A., Lachapelle, F., Lombrail, P. &Gumpel, M. (1988) Myelination by oligodendrocytes isolated from 4–6 week-old rat CNS and transplanted into newborn shiverer brain.Journal of the Neurological Sciences 88, 161–75.
Ludwin, S. K. (1981) Pathology of demyelination and remyelination.Advances in Neurology 31, 123–68.
Poltorac, M. &Freed, W. J. (1989) Immunological reactions induced by intracerebral transplantation: evidence that host microglia but not astroglia are the antigen-presenting cells.Experimental Neurology 103, 222–33.
Prineas, J. &Connell, F. (1979) Remyelination in multiple sclerosis.Annals of Neurology 5, 22–31.
Rosen, C. L., Bunge, R. P., Ard, M. D. &Wood, P. W. (1989) Type I astrocytes inhibit myelination by adult rat oligodendrocytesin vitro.Journal of Neuroscience 9, 3371–9.
Rosenbluth, J. (1976) Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axon of the frog brain.Journal of Neurocytology 5, 731–45.
Rosenbluth, J., Hasegawa, M. &Schiff, R. (1989) Myelin formation in myelin-deficient rat spinal cord following transplantation of normal fetal spinal cord.Neuroscience Letters 97, 35–40.
Rosenbluth, J., Hasegawa, M., Shirasaki, N., Rosen, C. L. &Liu, Z. (1990) Myelin formation following transplantation of normal fetal glia into myelin-cleficient rat spinal cord.Journal of Neurocytology 19, 718–30.
Rosenbluth, J., Liu, Z., Guo, D. &Schiff, R. (1991) Myelin formation by mouse glia in myelin-deficient rats treated with cyclosporine.Journal of Cell Biology 115, 214a.
Schnapp, B. &Mugnaini, E. (1975) The myelin sheath: electron microscopic studies with thin sections and freeze-fracture. InGolgi Centennial Symposium Proceedings (edited bySantini, M.), pp. 209–33. New York: Raven.
Sigal, N. H. &Dumont, F. J. (1992) Cyclosporin A, FK-506, and rapamycin: pharmacological probes of lymphocyte signal transduction.Annual Review of Immunology 10, 519–60.
Tao-Cheng, J.-H. &Rosenbluth, J. (1983) Axolemmal differentiation in myelinated fibers of rat peripheral nerves.Developmental Brain Research 9, 251–63.
Warrington, A. E., Barbarese, E. &Pfeiffer, S. E. (1993) Differential myelinogenic capacity of specific developmental stages of the oligodencrocyte lineage upon transplantation into hypomyelinating hosts.Journal of Neuroscience Research 34, 1–13.
Wolf, M. K., Brandenberg, M. &Billings-Gagliardi, S. (1986) Migration and myelination by adult glial cells: reconstructive analysis of tissue culture experiments.Journal of Neuroscience 6, 3731–88.
Wood, P. M. &Bunge, R. P. (1991) The origin of remyelinating cells in the adult central nervous system: the role of the mature oligodendrocyte.Glia 4, 225–32.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rosenbluth, J., Liu, Z., Guo, D. et al. Myelin formation by mouse glia in myelin-deficient rats treated with cyclosporines. J Neurocytol 22, 967–977 (1993). https://doi.org/10.1007/BF01218354
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01218354