Abstract
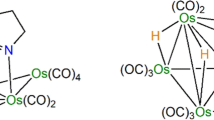
The reaction of the lightly stablized cluster [Os3(CO)10(NCMe)2] with thiosalicylic acid affords two products [{Os3(CO)10(µ-H}]2SC6H4CO2],1 and [Os3H(CO)10SC6,H4C(O)OOs3H(CO)11],2. Complex 2 undergoes CO dissociation to give1 or fragmentation to give [Os3H(CO)10SC6H4 COOH], 3 in solution. Reaction of phthalic acid and [ Os3(CO)10(NCMc)2] gives two products [{Os3(CO)10(µ-H)}2O2CC6H4CO2], 4 and [Os3H(CO)10O2CC6 H4C(O)OOs3H(CO)11], 5. 5 also undergoes CO dissociation to give4, but no such conversion is observed in the preparation of [{Os3(CO)10(µH)}2 (SC6H4S)],6 from the reaction betweeno-dithiobenzene and [Os3(CO)10 (NCMe)2]. Unlike thiosalicylic acid, treatment of [Os3(CO)10(NCMe)2] with 1 equivalent 2,2'-dithiosalicylaldehyde in dichloromethane produces the compounds [Os3(CO)10(SC6H4CHO)2],7 and [Os3(CO)10µ-H)(SC6H4CHO)].8 in moderate yields which are stable in both the solid state and solution. The mechanism for the formation of1-5 is also proposed. All the clusters1-8 have been fully characterized by conventional spectroscopic methods and the structures of1, 3, 4, 7, and8 have been established by X-ray, crystallography.
Similar content being viewed by others
References
K. H. Whitmire (1988).J. Coor. Chem. 17, 95: (b) K. H. Whitmire (1991).J. Cluster Sci. 2, 231.
D. V. Shriver, H. D. Kaesz, and R. D. Adams (eds.),The Chemistry of Metal Cluster plexes (VCH, New York, 1990), pp. 252–259.
R. D. Adams (1985).Polyhedron 4, 2003.
G. R. Frauenhoff (1992).Coord. Chem. Rev. 121, 131: (b) K. I. Hardcastle, T. McPhillips, A. J. Arce, Y. De Sanctis, A. I. Deeming, and N. I. Powell (1990).J. Organomet. Chem. 389, 361.
K. A. Azam, A. J. Deeming, and I. P. Rothwell (1981).J. Chem. Soc., Dalton Trans. 91.
D. L. Keiter, D. S. Strickland, S. R. Wilson and J. R. Shapley (1986)Am. Chem. Soc. 108, 3846.
G. D. Jarvinen and R. R. Ryan (1984).Organometallics 3, 1431.
G. R. Frauenhoff, S. R. Wilson, and J. R. Shapley (1991).Inorg. Chem. 30, 78.
S. M. Lee, K. K. Cheung, and W. T. Wong (1995).J. Organomet. Chem. 494, 273.
S. M. Lee, K. K. Cheung, and W. T. Wong (1995).J. Organomet. Chem. 503, C19.
I. S. M. Lee and W. T. Wong (1996).J. Clustcr Sci. 7, 37.
B. F. G. Johnson, J. Lewis, P. R. Raithby, V. P. Saharan, and W. T. Wong (1991).J. Chem. Soc. Chem. Commun. 365.
J. R. Shapley, G. M. St. George, M. R. Churchill, and F. J. Houander (1982)Inorg. Chem. 21, 3295.
B. F. G. Johnson, J. Lewis, P. R. Raithby, and W. T. Wong (1991).J. Organomet. Chem. 401, C50.
A. P. Humphries and H. D. Kaesz (1979).Prog. Inorg. Chem. 2, 145.
M. R. Churchill and B. G. DeBoer (1977).Inorg. Chem. 16, 878.
R. D. Adams. Z. Dawoodii, D. F. Foust, and B. E. Segmüller (1983).J. Am. Chem. Soc. 105. 831.
R. D. Adams, I. T. Horvath, and L. W. Yang (1983)J. Am. Chem. Soc. 105, 1533.
R. D. Adams and J. G. Wang (1989).Polyherdron 8, 1437.
M. G. Karlpor, S. P. Tunik, V. R. Denisor, G. L. Starova, A. B. Nikol'skii, F. M. Dolgushin, A. I. Yanovsky, and Y. T. Struchkov (1995)J. Organomet. Chem. 485, 219.
W. G. Y. Ho and W. T. Wong (1995).Polyhedron 14, 2849.
Y. K. Au, K. K. Cheung, and W. T. Wong (1995).Inorg. Chem. Acta 228, 267.
D. C. Brower, P. B. Winston, I. L. Tonker, and J. L. Templeton (1986).Inorg. Chem. 25, 2883.
G. B. Deach and R. J. Phillips (1980).Coord. Chem. Res. 33, 227.
R. Gobetto, K. I. Hardcastle, S. E. Kabir, L. Milone, N. Nishimura, M. Botta, E. Rosenberg, and M. Yin (1995).Organometallics,14, 3068.
A. G. Orpen (1981).J. Chem. Soc., Dalton Trans. 2509.
H. D. Holden, B. F. G. Johnson, J. Lewis, P. R. Railhby, and G. Uden (1983).Acta Crystallogr C39, 1203.
Y. K. Au. K. K. Cheung, and W. T. Wong (1995).J. Chem. Soc., Dalton Trans. 1047.
B. F. G. Johnson, T. M. Layer, J. Lewis. A. Martin, and P. R. Raithby (1992).J. Organometa. Chem. 429, C41.
T. M. Layer, J. Lewis, A. Martin, P. R. Raithby, and W. T. Wong (1992),J. Chem. Soc., Dalton Trans. 3411.
A. J. Arce, P. Arrojo, Y. De Sanctis, A. J. Deeming, and D. J. West (1992),Polyhedron 11, 1013.
A. J. Deeming, R. Vaish, A. J. Arce, and Y. De Sanctis (1994).Polyhedron 13, 3285.
H. D. Holden, B. F. G. Johnson, J. Lewis, P. R. Raithby, and G. Uden (1983).Acta Crystallogr. C39, 1200.
D. D. Perrin, W. L. F. Armarego, and D. R. Perrin,Purification of Laboratory Chemicals (Pergamon Press, Oxford, 1980.
J. N. Nicholls and M. D. Vargas (1989).Inorg Synth. 26, 289.
H. S. Kasmai and S. G. Mischkl (1989).Synthesis 763.
A. C. T. North, D. C. Phillips, and F. S. Mathews (1968).Acta Crystallogr. A24, 351.
M. C. Byrla, M. Camalli, G. Cascarano, C. Giacovazzo, G. Polidori, R. Spagna, and D. Viterbo (1989).J. Appl. Crystallogr. 22, 389.
A. Altomare, M. Cascarano, C. Giacovazzo, A. Guagliardi, M. C. Burla, G. Polidori, and M. Camalli (1994).J. Appl. Crystallogr. 27, 435.
TEXSAN: Crystal Structure Analysis Package, Molecular Structure Corporation, The Woodlands, TX. 1985 and 1992.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, SM., Cheung, K.K. & Wong, WT. Triosmium carbonyl clusters of sulfur and oxygen mixed donor ligands. J Clust Sci 7, 435–453 (1996). https://doi.org/10.1007/BF01171193
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01171193