Abstract
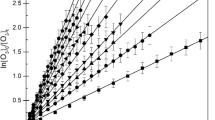
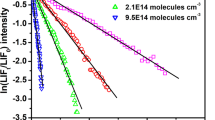
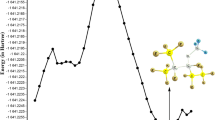
Chlorine atom-initiated photooxidations of CH2FCF3 (HFC-134a) in O2-N2 diluent were carried out to identify the products formed from the\(CF_3 CHF\dot O\) radical reactions and to determine the product yields as a function of temperature, pressure and O2 concentration. CF3C(O)F and HC(O)F were the major ‘first-generation’ products observed, along with smaller yields of C(O)F2 and, as yet, undetermined yields of CF3OOOCF3 and CF3OOC(O)F. The relative importance of the two major\(CF_3 CHF\dot O\) reaction pathways,
is expressed by the rate constant ratio
The decomposition reaction leading to HC(O)F and ĊF3 radical products is predicted to be the dominant pathway at the Earth's surface while mainly CF3C(O)F formation will occur at the tropopause.
Similar content being viewed by others
References
Anderson, L. R. and Fox, W. B., 1967, An unusual reaction of oxygen difluoride. The addition to carbonyl fluoride to produce bis(trifluoromethyl) trioxide,J. Amer. Chem. Soc. 89, 4313–4315.
Atkinson, R., 1990, Tropospheric reactions of the haloalkyl radicals formed from hydroxyl radical reaction with a series of alternative fluorocarbons, pp. 165–205 inScientific Assessment of Stratospheric Ozone: 1989, World Meteorological Organization Global Ozone Research and Monitoring Project — Report No. 20, Volume II, Appendix: AFEAS Report, Geneva, Switzerland.
Atkinson, R., Baulch, D. L., Cox, R. A., Hampson, R. F. Jr., Kerr, J. A., and Troe, J., 1992, Evaluated kinetic and photochemical data for atmospheric chemistry: Supplement IV,J. Phys. Chem. Ref. Data 21, 1125–1568.
Cauble, R. L. and Cady, G. H., 1968, Fluorocarbonyl trifluoromethylperoxide,J. Org. Chem. 33, 2099–2100.
Derwent, R. G. and Volz-Thomas, A., 1990, The tropospheric lifetimes of halocarbons and their reactions with OH radicals: an assessment based on the concentration of14CO, pp. 127–146 inScientific Assessment of Stratospheric Ozone: 1989, World Meteorological Organization Global Ozone Research and Monitoring Project — Report No. 20, Volume II; Appendix: AFEAS Report, Geneva, Switzerland.
Hirschmann, R. P., Fox, W. B., and Anderson, L. R., 1969, The infrared spectrum of bis(trifluoromethyl) trioxide,Spectrochim. Acta 25A, 811–817.
McLinden, M. O. and Didion, D. A., 1989, Thermophysical-property needs for the environmentally acceptable halocarbon refrigerants,Int. J. Thermophys. 10, 563–576.
Sehested, J. and Wallington, T. J., 1993, Atmospheric chemistry of hydrofluorocarbon 134a; fate of the alkoxy radical CF3O,Environ. Sci. Technol. 27, 146–152.
Sukornick, B., 1989, Potentially acceptable substitutes for the chlorofluorocarbons: properties and performance features of HFC-134a, HCFC-123, and HCFC-141b,Int. J. Thermophys. 10, 553–561.
Thompson, P. G., 1967, Bis(perfluoroalkyl) trioxides,J. Am. Chem. Soc. 89, 4316–4319.
Tuazon, E. C. and Atkinson, R., 1993, Tropospheric transformation products of a series of hydrofluorocarbons and hydrochlorofluorocarbons,J. Atmos. Chem., submitted for publication.
Wallington, T. J., Hurley, M. D., Ball, J. C., and Kaiser, E. W., 1992, Atmospheric chemistry of hydrofluorocarbon 134a: Fate of the alkoxy radical CF3CFHO,Environ. Sci. Technol. 26, 1318–1324.
Winer, A. M., Graham, R. A., Doyle, G. J., Bekowies, P. J., McAfee, J. M., and Pitts, J. N. Jr., 1980, An evacuable environmental chamber and solar simulator facility for the study of atmospheric photo-chemistry,Adv. Environ. Sci. Technol. 10, 461–511.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tuazon, E.C., Atkinson, R. Tropospheric degradation products of CH2FCF3 (HFC-134a). J Atmos Chem 16, 301–312 (1993). https://doi.org/10.1007/BF01032627
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01032627