Summary
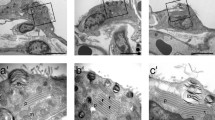
The nature of Cuprolinic Blue-positive anionic filaments in mouse lung alveoli has been characterized. The contrast of filaments in the alveolar basement membrane of type I epithelial cells was lost on treatment with nitrous acid and pronase (without prefixation). In contrast, neither neuraminidase, chondroitinase ABC or AC, norStreptomyces hyaluronidase had any effect. Treatment with pronase (after prefixation) and 2.0m MgCl2 (after prefixation) also had no effect, indicating that the filaments are heparan sulphate proteoglycans. The filaments in the alveolar basement membrane of type II epithelial cells and in the capillary basement membrane of the endothelial cells were also nitrous acid sensitive, but chondroitinase ABC-insensitive. A model in which the whole alveolus contains a single layer of heparan sulphate-containing proteoglycan monomers is proposed. Furthermore, the collagen fibril associated filaments remained unaffected after treatment with nitrous acid, neuraminidase orStreptomyces hyaluronidase, or after digestion with pronase (after prefixation) and treatment with 2.0m MgCl2 (after prefixation). These filaments, however, could no longer be detected when digestion with chondroitinase ABC or pronase (without prefixation) was applied; chondroitinase AC treatment clearly affected the filaments, although they still were visible. These results indicate that the filaments are dermatan sulphate-containing proteoglycans. Some functional aspects of the proteoglycans are discussed.
Similar content being viewed by others
References
Ausprunk, D. H., Boudreau, C. L. &Nelson, D. A. (1981) Proteoglycans in the microvasculature. I: Histochemical localization in microvessels of the rabbit eye.Am. J. Path. 103, 353–66.
Cantor, J., Parshley, M. S., Darnule, A. T., Mandl, I. &Turino, G. M. (1977) Glycosaminoglycan synthesis by a clone of rat lung endothelial cells.J. Cell Biol. 75, 75a.
Castor, C. W., Heiss, P. R., Gray, R. M. &Seidman, J. C. (1979) Connective tissue formation by lung fibroblasts in vitro.Am. Rev. Respir. Dis. 120, 107–19.
Cheung, D. T. &Nimni, M. E. (1982), Mechanism of crosslinking of proteins by glutaraldhyde. I: Reaction with model compounds; II: Reaction with monomeric and polymeric collagen.Conn. Tiss. Res. 10, 187–216.
Clowes, A. W., Clowes, M. M., Gown, A. M. &Wight, T. N. (1982) Distribution of a proteoheparan sulfate in rat aorta.Fedn. Proc. 41, 441.
Danielsen, C. C. (1982) Mechanical properties of reconstituted collagen fibrils. Influence of a glycosaminoglycan: dermatan sulfate.Conn. Tiss. Res. 9, 219–25.
Ehrlich, K. C. (1981) Proteoglycans synthesis by rat lung cells culturedin vitro.J. biol. Chem. 256, 73–80.
Eyre, D. R. &Muir, H. (1975) The distribution of different molecular species of collagen in fibrous, elastic and hyaline cartilages of the pig.Biochem. J. 151, 595–602.
Farin, F., Killen, P. &Striker, G. (1980) Biosynthesis of heparan sulfate (glycosaminoglycans) by glomerular cellsin vitro.Fedn. Proc. 39, 873.
Farquhar, M. G. &Kanwar, Y. S. (1982) Functional organization of the glomerulus. InImmune Mechanism in Renal Disease (edited byCummings, N. B., Michael, A. F. andWilson, C. B.) New York: Plenum Press.
Geyer, G. (1973)Ultrahistochemie, 2nd edn., pp. 13–22. Stuttgart: Gustav Fischer Verlag.
Greenwald, R. A., Schwartz, C. E. &Cantor, J. O. (1975) Interaction of cartilage proteoglycans with collagen-substituted agarose gels.Biochem. J. 145, 601–05.
Hassell, J. R., Robey, P. G., Barrach, H.-J., Wilczek, J., Rennard, S. I. &Martin, G. R. (1980) Isolation of a heparan sulfate-containing proteoglycan from basement membrane.Proc. Natn. Acad. Sci. U.S.A. 77, 4494–8.
Horwitz, A. L. &Crystal, R. G. (1975) Content and synthesis of glycosaminoglycans in the developing lung.J. clin. Invest. 56, 1312–8.
Horwitz, A. L., Elson, N. A. &Crystal, R. G. (1976) Proteoglycans and elastic fibres. InThe Biochemical Basis of Pulmonary Function; Lung Biology in Health and Disease (edited byCrystal, R. G.), Vol. 2, pp. 273–311. New York, Basel, Marcel Dekker, Inc.
Junqueira, L. C. U., Toledo, O. M. S. &Montes, G. S. (1981) Correlation of specific sulfated glycosaminoglycans with collagen types I, II and III.Cell Tiss. Res. 217, 171–5.
Kanwar, Y. S. &Farquhar, M. G. (1979) Presence of heparan sulfate in the glomerular basement membrane.Proc. Natn. Acad. Sci. U.S.A. 76, 1303–7.
Kanwar, Y. S. &Farquhar, M. G. (1980) Role of glycosaminoglycans (GAG) in the permeability of the glomerular basement membrane. (GBM).Fedn. Proc. 39, 334.
Kanwar, Y. S., Linker, A. &Farquhar, M. G. (1980) Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion.J. Cell Biol. 86, 688–93.
Kanwar, Y. S., Hascall, V. C. &Farquhar, M. G. (1981) Partial characterization of newly synthesized proteoglycans isolated from the glomerular basement membrane.J. Cell Biol. 90, 527–32.
Kawamoto, T. &Nagai, Y. (1976) Developmental changes in glycosaminoglycans, collagen and collagenase activity in embryonic chick skin.Biochim. biophys. Acta 437, 190–9.
Kosher, R. A. &Searls, R. L. (1973) Sulfated mucopolysaccharide synthesis during the development ofRana pipiens.Devl Biol. 32, 50–68.
Lindahl, U. &Höök, M. (1978) Glycosaminoglycans and their binding to biological macromolecules.Ann. Rev. Biochem. 47, 385–417.
Linker, A. &Hovingh, P. (1972) Heparinase and heparitinase from flavobacteria.Meth. Enzym. 28, 902–11.
Maeda, H., Ishikawa, H. &Ohta, S. (1981) Circumscribed myxoedema of lichen myxoedematosus as a sign of faulty formation of the proteoglycan macromolecule.Br. J. Derm. 105, 239–45.
Mathews, M. B. (1965) The interaction of collagen and acid mucopolysaccharides. A model for connective tissue.Biochem. J. 96, 710–6.
Matthay, M. A., Landolt, C. C. &Staub, N. C. (1982) Differential liquid and protein clearance from the alveoli of anesthetized sheep.J. appl. Physiol. 53, 96–104.
Nakamura, N., Kojima, J., Okamoto, S. &Kitamura, Y. (1981) Absence of heparin in glycosaminoglycan fractions isolated from the skin of genetically mast cell-depletedW/W V mice.Biochem. Internat. 3, 449–56.
Öbrink, B. (1973a) A study of the interactions between monomeric tropocollagen and glycosaminoglycans.Eur. J. Biochem. 33, 387–400.
Öbrink, B. (1973b) The influence of glycosaminoglycans on the formation of fibres from monomeric tropocollagenin vitro.Eur. J. Biochem. 34, 129–37.
Olver, R. E., Ramsden, C. A. &Strong, L. B. (1981) Adrenaline-induced changes in net lung liquid volume flow across the pulmonary epithelium of the fetal lamb: evidence for active sodium transport.J. Physiol. 319, 38P-39P.
Oohira, A., Wight, T. N., McPherson, J. &Bornstein, P. (1982) Biochemical and ultrastructural studies of proteoheparan sulfates synthesized by PYS-2, a basement membrane-producing cell line.J. Cell Biol. 92, 357–67.
Parthasarathy, N. &Spiro, R. G. (1982) Basement membrane glycosaminoglycans: examination of several membranes and evaluation of the effect of sonic treatment.Archs Biochem. Biophys. 213, 504–11.
Radhakrishnamurthy, B., Smart, F., Dalferes, E. R. &Berenson, G. S. (1980) Isolation and characterization of proteoglycans from bovine lung.J. biol. Chem. 255, 7575–82.
Sampson, P. M., Jimenez, S. A. &Bashey, R. I. (1979) Isolation and partial characterization of proteoglycans from sheep lung parenchyma.Biochim. biophys. Acta 588, 129–41.
Schmid, K., Grundboeck-Jusco, J., Kimura, A., Tschopp, F. A., Zollinger, R., Binette, J. P., Lewis, W. &Hayashi, S. (1982) The distribution of the glycosaminoglycans in the anatomic components of the lung and the changes in concentration of these macromolecules during development and aging.Biochim. biophys. Acta 716, 178–87.
Scott, J. E. (1972) Histochemistry of Alcian Blue. III: The molecular biological basis of staining by Alcian Blue 8GX and analogous phthalocyanins.Histochemie 32, 191–212.
Scott, J. E., Orford, C. R. &Huges, E. W. (1981) Proteoglycan-collagen arrangements in developing rat tail tendon. An electron-microscopical and biochemical investigation.Biochem. J. 195, 573–81.
Snowden, J. McK. &Swann, D. A. (1980) Effects of glycosaminoglycans and proteoglycans on thein vitro assembly and thermal stability of collagen fibrils.Biopolymers 19, 767–80.
Stefanovich, V. &Gore, I. (1967) A micromethod for the determination of acid mucopolysaccharides in vascular tissue.J. Chromat. 31, 473–8.
Straus, A. H., Nader, H. B. &Dietrich, C. P. (1982) Absence of heparin-like compounds in mast-cell-free tissue and animals.Biochim. biophys. Acta 717, 478–85.
Toole, B. P. &Lowther, D. A. (1968) Dermatan sulfate-protein: Isolation from and interaction with collagen.Archs Biochem. Biophys. 128, 567–78.
Vaccaro, C. A. &Brody, J. S. (1979) Ultrastructural localization of proteoglycans in the pulmonary alveolus.Am. Rev. Respir. Dis. 120, 901–10.
Vaccaro, C. A. &Brody, J. S. (1981) Structural features of alveolar wall basement membrane in the adult rat lung.J. Cell Biol. 91, 427–37.
Vogel, K. G. &Peterson, D. W. (1981) Extracellular, surface, and intracellular proteoglycans produced by human embryo lung fibroblast in culture. (IMR-90).J. biol. Chem. 256, 13235–42.
Wusteman, F. S. (1972) Glycosaminoglycans of bovine lung parenchyma and pleura.Experientia 28, 887–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Kuppevelt, T.H.M.S.M., Cremers, F.P.M., Domen, J.G.W. et al. Staining of proteoglycans in mouse lung alveoli. II. Characterization of the Cuprolinic Blue-positive, anionic sites. Histochem J 16, 671–686 (1984). https://doi.org/10.1007/BF01003394
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01003394