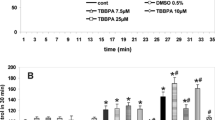
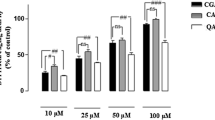
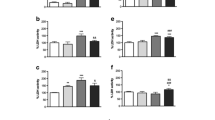
Abstract
In our previous experiments, evidence of free radical formation has been demonstrated in gerbil brain after kainic acid (KA) administration. In the present study, the mechanisms involved in KA-induced free radical formation and subsequent cell degeneration were investigated using high density cortical neuron cultures. A free radical trapping agent,a-phenyl-N-tert-butyl-nitrone (PBN), as well as the combined action of superoxide dismutase and catalase attenuated KA neurotoxic effect. Calpain-induced xanthine oxidase (XO) activation may play an important role in KA excitotoxicity since calpain inhibitor I as well as allopurinol, a selective XO inhibitor, significantly protected the cortical neurons from KA-induced cell death. However, XO activation may not be the only source producing free radicals, other free radical generating systems such as nitric oxide synphase may also play a role in KA insult.
Similar content being viewed by others
References
Rothman, S. M., and Olney, J. W. 1987. Excitotoxicity and the NMDA receptor. Trends Neurosoci. 10:229–302.
Young, A. B. 1991. Roles of Excitotoxins in heredito-degenerative neurological diseases. Research Publication — Association for Research in Nervous & Mental Diseases 71:175–89.
Choi, D. W. 1989. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1:623–34.
Hayes, R. L., Jenkins, L. W., and Lyeth, B. G. 1992. Neurotransmitter-mediated mechanisms of traumatic brain injury: Acetylcholine and excitatory amino acids. J. Neurotrauma 9(Suppl. 1):S173–87.
Beal, M. F., Kowall, N. W., Ellison, N. W., Mazurek, M. F., Swartz, K. J., and Martin, J. B. 1986. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature 321:168–71.
Watkins, K. C., Curtis, D. R., and Biscoe, T. J. 1966. Central effects of b-N-oxalyl-a,b-diaminopropionic acid and other lathyrus factors. Nature 211:637.
Olney, J. W., Misra, C. H., and Rhee, V. 1976. Brain and retinal damage from lathyrus excitotoxin, b-N-oxalyl-L-a,b-diaminopropionic acid. Nature 264:659–61.
Weiss, J. H., and Choi, D. W. 1990. Slow non-NMDA receptor mediated neurotoxicity and amyotrophic lateral sclerosis. Advance Neurol. 56:311–8.
Davies, P., Katzman, R., and Terry, R. D. 1980. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer's disease and Alzheimer's senile dementia. Nature 288:279–80.
Koh, J-Y., Goldberg, M. P., Hartley, D. M., and Choi, D. W. 1990. Non-NMDA receptor-mediated neurotoxicity in cortical culture. J. Neurosci. 10:693–705.
Kato, K., Puttfarcken, P. S., Lyons, W. E., and Coyle, J. T. 1991. Developmental time course and ionic dependence of kainate-mediated toxicity in rat cerebellar granule cell cultures. J. Pharm. Exp. Therap. 265:402–11.
Schousboe, A., Frandsen, A., and Kregsgaard-Larsen, P. 1992. Pharmacological and functional characterization of excitatory amino acid mediated cytotoxicity in cerebral cortical neurons. Cell. Biol. & Toxicology 8:93–100.
Rothman, S. M., Thurston, J. H., and Hauhart, R. E. 1987. Delayed neurotoxicity of excitatory amino acids in vitro. Neurosci. 22:471–80.
MacDermott, A. B., Mayer, M. L., Westbrook, G. L., Smith, S. J., and Barker, J. L. 1986. NMDA receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurons. Nature 321:519–22.
Garthwaite, G., and Garthwaite, J. 1986. Neurotoxicity of excitatory amino acid receptor agonists in rat cerebellar slices: dependence of calcium concentration. Neurosci. Lett. 66:193–8.
Weiss, J. H., Hartley, D. M., Koh, J., and Choi, D. W. 1990. The calcium channel blocker nifedipine attenuates slow excitatory amino acid neurotoxicity. Science 247:1474–7.
Kontos, H. A., Wei, E. P., Povlishock, T. J., Dietrich, W. D., Margiera, C. J., and Ellis, E. F. 1980. Cerebral arteriolar damage by arachidonic acid and prostaglandin G2. Science 209:1242–4.
Lynch, M. A., and Bliss, T. V. P. 1986. Long term potentiation of synaptic transmission in the hippocampus of the rat; effect of calmodulin and oleoyl-acetyl-glycerol on release of3H-glutamate. Neurosci. Lett. 65:171–6.
Connor, J. A., Wadman, W. J., Hockberger, P. E., and Wong, R. K. S. 1988. Sustained dentritic gradients of Ca2+ induced by excitatory amino acids in CA1 hippocampus neurons. Science 240:649–53.
Orrenius, S., McConkey, D. J., Bellomo, G., and Nicotera, P. 1989. Role of Ca2+ in toxic cell killing. Trends Pharmacol. Sci. 10:281–5.
Garthwaite, J., Charles, S. L., and Chess-Williams, R. 1988. Endothelium-derived relaxing factor release on activation of the NMDA receptors suggests role as intercellular messenger in the brain. Nature 336:385–6.
Melloni, E., and Portremoli, S. 1989. The calpains. Trends Neurosci. 12:438–44.
Orrenius, S., Burkitt, M. J., Kass, G. E., Dypbukt, J. M., and Nicotera, P. 1992. Calcium ions and oxidative cell injury. Annals Neurol. 32(Suppl.):S33–42.
Sun, A. Y., Cheng, Y., Bu, Q., and Oldfield, F. 1992. The Biochemical mechanisms of the excitotoxicity of kainic acid. Mol. Chem. Neuropath. 17:51–63.
Dichter, A. 1978. Rat cortical neurons in cell culture: Culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 149:279–93.
Bergmeyer, H. U., and Bernt, E. 1974. Lactate Dehydrogenase, in Methods of Enzymatic Analysis. (Bergmeyer, HU. eds. 2nd. ed.), Academic Press, Inc., New York & London.
McCord, J. M. 1987. Oxygen-derived radicals: A link between reperfusion injury and inflammation. Fed. Proc. 46:2402–6.
Coyle, J. T., and Puttfarcken, P. 1993. Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–95.
Giffard, R. G., Bruno, V. M. G., Amagasu, S. M., Choi, D. W., and Dugan, L. L. 1992. NMDA receptor activation results in hydroxl radical production in primary murine cortical cultures. Soc Neurosci (Abstracts) 18:646.
Chan, P. H., Chu, L., Chen, S. F., Carlson, E. J., and Epstein, C. J. 1990. Reduced neurotoxicity in transgenic mice overexpressing human copper-zinc-superoxide dismutase. Stroke 21(suppl. 3):80–82.
Zweier, J. L., Kuppusamy, P., Thompson-Gorman, S., Klunk, D., and Lutty, G. A. 1994. Measurement and characterization of free radical generation in reoxygenated human endothelial cells. Am. J. Physiol. 266:C700-C708.
Lipton, S. A., and Rosenberg, P. 1994. Excitatory amino acids as a final common pathway for neurologic disorders. New Engl. J. Med. 330:613–22.
Ahmad, F. F., Cowan, D. L., and Sun, A. Y. 1989. Spin trapping studies of the influence of alcohol on lipid peroxidation. In “Molecular Mechanisms of Alcohol” (G. Y. Sun, P. K. Rudeen, W. G. Wood, Y. H. Wei, and A. Y. Sun, eds) Humana Press, Clifton, NJ., pp. 257–78.
Carney, J. M., and Floyd, R. A. 1991. Protection against oxidative damage to CNS by a-phenyl-tert-butyl nitrone (PBN) and other spin-trapping agents: a novel series of nonlipid free radical scavenger. J. Molecular Neurosci. 3:47–57.
Choi, D. W. 1992. Excitotoxic cell death. J. Neurobiol. 23:1261–76.
Goldberg, M. P., Gifford, R. G., Kurth, M. C., and Choi, D. W. 1989. Role of extracellular calcium and magnesium in ischemic neuronal injury in vitro. Neurology 39(suppl. 1):217.
Michaels, R. L., and Rothman, S. M. 1990. Glutamate neurotoxicity in vitro: Antagonist pharmacology and intracellular calcium concentrations. J. Neurosci. 10:283–92.
Sun, A. Y., Cheng, Y., and Sun, G. Y. 1992. Kainic acid-induced excitotoxicity in neurons and glial cells. Prog. Brain Res. 94:271–80.
Dumuis, a., Sebben, M., Haynes, L., Pin, L-P., and Bockaert, J. 1988. NMDA receptors activate the arachidonate cascade system in striatal neurons. Nature 336:68–70.
Lazarewicz, J. W., Wroblewski, J. T., and Costa, E. 1990. NMDA-sensitive glutamate receptors induce calcium-mediated arachidonic acid release in primary cultures of cerebellar granule cells. J. Neurochem. 55:1875–81.
Seubert, P. Larson, J., Oliver, M., Jung, M. W., Baudry, M., and Lynch, G. 1988. Stimulation of NMDA receptors induces proteolysis of spectrin in hippocampus. Brain Res. 459:233–40.
Siman, R., and Noszek, J. C. 1988. Excitatory amino acids activate calpain and induce structure protein breakdown in vivo. Neuron. 1:279–87.
Arai, A., Kessler, M., Lee, K., and Lynch, G. 1990. Calpain inhibitors improve the recovery of synaptic transmission from hypoxia in hippocampal slices. Brain Res. 532:63–8.
Manev, H., Favaron, M., Siman, R., Guidotti, A., and Costa, E. 1991. Glutamate neurotoxicity is independent of calpain I inhibition in primary cultures of cerebellar granule cells. J. Neurochem. 57:1288–95.
Dawson, V. L., Dawson, T. M., London, E. D., Bredt, D.S., and Snyder, S. H. 1991. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. USA 88:6368–74.
Buttelli, M. G., Lorenzoni, E., and Stirpe, F. 1973. Milk xanthine oxidase type D (dehydrogenase) and type O (oxidase). Biochem. J. 131:191–98.
Retz, K. C., and Coyle, J. T. 1982. The effects of kainic acid on high energy metabolites in the mouse striatum. J. Neurochem. 38:196–203.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cheng, Y., Sun, A.Y. Oxidative mechanisms involved in kainate-induced cytotoxicity in cortical neurons. Neurochem Res 19, 1557–1564 (1994). https://doi.org/10.1007/BF00969006
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00969006