Abstract
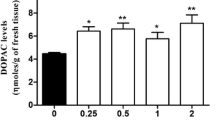
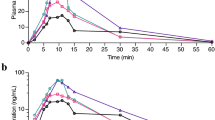
Tetrahydrobiopterin, the hydroxylase cofactor (BH4) was administered (i.v. 20 mg/kg) to Rhesus monkeys. Within 90 min of its administration CSF cofactor levels increased significantly above baseline levels. Peak CSF levels were attained at 90–180 min time period following cofactor injection and returned to baseline gradually over the next 15 hrs. The increased brain cofactor levels had no apparent effect on synthesis of dopamine, norepinephrine or serotonin as evidenced by a lack of change in the levels of the metabolites homovalillic acid, 3-methoxy-4-hydroxyphenyleneglycol, and 5-hydroxyindoleacetic acid. The present results
Similar content being viewed by others
Abbreviations
- BH4 :
-
tetrahydrobiopterin
- CSF:
-
cerebrospinal fluid
- 5-HIAA:
-
5-hydroxyindoleacetic acid
- HAV:
-
homovanillic acid
- MHPG:
-
3-methoxy-4-hydroxyphenyleneglycol
References
Williams, A. C., Eldridge, R., Levine, R. A., Lovenberg, W., andPaulson, G.: Low CSF hydroxylase cofactor (tetrahydrobiopterin) levels in inherted dystonia. Lancet 2:8139:410–411, 1979.
Nagatsu, T. 1981. Biopterin cofactor and regulation of monoamine-synthesizing monooxygenases. Trends in Pharmacol. Sci. 2:276–279.
Kaufman, S. 1959. Studies on the mechanism of the enzymatic conversion of phenylalanine to tyrosine. J. Biol. Chem. 234:2677–2682.
Nagatsu, T., Levitt, M., andUdenfriend, S. 1964. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J. Biol. Chem. 239:2910–2917.
Lovenberg, W., andBruckwick, E. A. 1975. Mechanisms of receptor mediated regulation of catecholamine synthesis in brain. Pages 149–169,Usdin, E. andBunney, W. E. (eds.),in Pre- and Post-synaptic Receptors, Presented at the American College of Neuropsychopharmacology, Puerto Rico, December 1974, Marcel Dekker.
Kettler, R., Bartholini, G., andPletscher, A. 1974. In vivo enhancement of tyrosine hydroxylation in rat striatum by tetrahydrobiopterin. Nature 249:476–478.
Patrick, R. L. andBarchas, J. D. 1976. Dopamine synthesis in rat brain striatal synaptosomes. II. Dibutyryl cyclic adenosine 3′:5′-monophosphoric acid and 6-methytetrahydropterin-induced synthesis increases without an increase in endogenous dopamine release. J. Pharmacol. Exp. Ther. 197:97–104.
Pollock, R. J., Kapatos, G. andKaufman, S. 1981. Effect of cyclic AMP-dependent protein phosphorylating conditions on the pH-dependent activity of tyrosine hydroxylase from beef and rat striata. J. Neurochem. 37:855–860.
Levine, R. A., Williams, A. C., Robinson, D. S., Calne, D. B., andLovenberg, W. 1979. Analysis of hydroxylase cofactor activity in the cerebrospinal fluid of patients with Parkinson's disease. Advances in Neurol. 24:303–307.
Williams, A. C., Levine, R. A., Chase, T. N., Lovenberg, W., andCalne, D. B. 1980. CSF hydroxylase cofactor levels in some neurological diseases. J. Neurol. Neurosurg. Psychiatry 43:735–738.
Bailey, S. W., andAyling, J. E. 1978. Separation and properties of the 6-diastereoisomers of Ferythrotetrahydrobiopterin and their reactivities with phenylalanine hydroxylase. J. Biol. Chem. 253:1598–1605.
Fukushima, T., andNixon, J. C. 1980. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal. Biochem. 102:176–188.
Scheinin, M., Chang, W. H., Kirk, K. L., andLinnoila, M. 1983. Simultaneous determination 3-methoxy-4-hydroxyphenylglycol, 5-hydroxyindoleacetic acid, and homovanillic acid in cerebrospinal fluid with high-performance Liquid chromatography using electrochemical detection. Anal. Biochem. 131:246–253.
Bradbury, M. 1979. Pages 121–125.in The Concept of a Blood-Brain Barrier. J. Wiley and Sons, Chichester, N.Y.
Brautigam, M., Dreesen, R. andHerken, H. 1984. Tetrahydrobiopterin and total biopterin content of neuroblastoma (NIE-115, NZA) and pheochromocytoma (PC-12) clones and the dependence of catecholamine synthesis on tetrahydrobiopterin concentration in PC-12 cells. J. Neurochem. 42:390–396.
Miller, L. P., andLovenberg, W. 1985. The use of the natural cofactor, (6R)-Lerythrotetrahydrobiopterin in the analysis of nonphosphorylated and phosphorylated rat striatal tyrosine hydroxylase at pH 7.0. Neurochem. Int., 7:689–697.
Author information
Authors and Affiliations
Additional information
Supported by Dystonia Medical Research Foundation, 9615 Brighton Way, Suite 416, Beverly Hills, California 90210
Rights and permissions
About this article
Cite this article
Miller, L., Insel, T., Scheinin, M. et al. Tetrahydrobiopterin administration to rhesus macaques. Neurochem Res 11, 291–298 (1986). https://doi.org/10.1007/BF00967976
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00967976