Summary
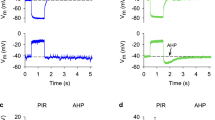
We have recorded fromParamecium a membrane depolarization in response to heat. This heat-induced depolarization is graded with the magnitude of the temperature change and can trigger action potentials. The mechanism for the Ca-action potential, localized in the cilia, is not needed in generating the heat-induced depolarization, since a ciliary Ca-channel mutant (pwB) and a deciliated wild type both show the same magnitude of depolarization as an intact wild type.
The direction and magnitude of change in the membrane potential in response to heat is affected by the initial level of the membrane potential. Conditions which depolarize the wild type decrease both the heat-induced depolarization and thermal avoidance behavior. A mutant with defective thermal avoidance behavior,teaB, is naturally less polarized at rest (Satow and Kung 1981) but can produce heat-induced depolarizations equal to that of the wild type if hyperpolarized to the wild-type levels by injection of constant current. Both the mutant and the wild type have apparent reversal potentials at −5 to −20 mV in 1 mmol/l Ca2+, above which the response to heat becomes a hyperpolarization. In both the wild type andteaB, the size of the heat-induced depolarization parallels the strength of the behavioral response to heat as measured by individual or population assays of thermal avoidance.
Similar content being viewed by others
References
Baldino F, Geller HM (1982) Electrophysiological analysis of neuronal thermosensitivity in rat preoptic and hypothalamic tissue cultures. J Physiol (Lond) 327:173–184
Carpenter DO (1981) Ionic and metabolic bases of neuronal thermosensitivity. Fed Proc 40:2808–2813
Chiu SY, Mrose HE, Ritchie JM (1979) Anomalous temperature dependence of the sodium conductance in rabbit nerve compared with frog nerve. Nature 279:327–328
Croll NA (1967) Acclimatization in the eccritic thermal response ofDitylenchus dipsaci. Nematologica 13:385–389
Dunlap K (1977) Localization of calcium channels inParamecium caudatum. J Physiol (Lond) 271:119–134
Eckert R, Brehm P (1979) Ionic mechanisms of excitation in Paramecium. Annu Rev Biophys Bioeng 8:353–383
Farrell J, Rose A (1967) Temperature effects on microorganisms. Annu Rev Microbiol 21:101–120
Georgescauld D, Duclohier H (1978) Transient fluorescence signals from pyrene labeled pike nerves during action potential. Possible implications for membrane fluidity changes. Biochem Biophys Res Commun 85:1186–1191
Guttman R (1971) The effect of temperature on the function of excitable membranes. In: Aldeman WJ Jr (ed) Biophysics and physiology of excitable membranes. Van Nostrand Reinhold, New York, pp 320–336
Hedgecock EM, Russel RL (1975) Normal and mutant thermotaxis in the nematodeCaenorhabditis elegans. Proc Natl Acad Sci USA 72:4061–4065
Hennessey TM (1981) Membrane lipids ofParamecium and their roles in sensory transduction. Ph D thesis, University of Wisconsin, Madison
Hennessey TM, Nelson DL (1979) Thermosensory behavior inParamecium tetraurelia: a quantitative assay and some factors that influence thermal avoidance. J Gen Microbiol 112:337–347
Hennessey TM, Nelson DL (1983) Biochemical studies of the excitable membranes ofParamecium tetraurelia. VIII. Temperature-induced changes in lipid composition and in thermal avoidance behavior. Biochim Biophys Acta 728:145–158
Hensel H (1973) Cutaneous thermoreceptors. In: Iggo A (ed) Somatosensory system. (Handbook of sensory physiology, vol II). Springer, Berlin Heidelberg New York, pp 79–110
Hensel H (1974) Thermoreceptors. Annu Rev Physiol 36:233–249
Hodgkin AL, Katz B (1949) The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol (Lond) 109:240–249
Jennings HS (1906) Behavior of the lower organisms. Indiana University Press, Bloomington, Indiana
Joyner RW (1981) Temperature effects on neuronal elements. Fed Proc 40:2814–2818
Kimelberg HK (1977) The influence of membrane fluidity on the activity of membrane-bound enzymes, In: Poste G, Nicolson GL (eds) Cell surface reviews, vol 3. North-Holland, New York, pp 205–293
Kung C (1979) The biology and genetics ofParamecium behavior. In: Breakfield XO (ed) Neurogenetics: genetic approaches to the nervous system. Oxford, New York, pp 1–26
Kung C, Saimi Y (1982) The physiological basis of taxes inParamecium. Annu Rev Physiol 44:519–534
Landowne D, Scruggs V (1976) The temperature dependence of the movement of potassium and chloride ions associated with nerve impulses. J Physiol (Lond) 259:145–158
Maeda K, Imae Y (1979) Thermosensory transduction inEscherichia coli: Inhibition of the thermoresponse by L-serine. Proc Natl Acad Sci USA 76:91–95
Mason P, Hasan H, Valis M (1978) Spontaneous firing of hypothalamic neurones over a narrow temperature interval. Nature 273:242–243
Naitoh Y (1968) Ionic control of the reversal response of cilia inParamecium caudatum. A calcium hypothesis. J Gen Physiol 51:85–103
Naitoh Y, Eckert R (1968) Electrical properties ofParamecium caudatum: Modification by bound and free cations. Z Vergl Physiol 61:427–452
Naitoh Y, Eckert R (1972) Electrophysiology of ciliate protozoa. In: Kerkut GA (ed) Experiments in physiology and biochemistry, vol V. Academic Press, London, pp 17–38
Nakaoka Y, Oosawa F (1977) Temperature-sensitive behavior ofParamecium caudatum. J Protozool 24:575–580
Nelson DL, Kung C (1978) Behavior ofParamecium: Chemical, physiological and genetic studies. In: Hazelbauer GL (ed) Taxis and behavior. Chapman and Hall, London, pp 75–100
Nelson DO, Prosser CL (1981) Intracellular recordings from thermosensitive preoptic neurons. Science 213:787–789
Nutik SL (1973) Posterior hypothalamic neurons responsive to preoptic region thermal stimulation. J Neurophysiol 36:238–249
Ogura A, Machemer H (1980) Distribution of mechanoreceptor channels in theParamecium surface membrane. J Comp Physiol 135:233–242
Romey G, Chicheportiche R, Lazdunski M (1980) Transition temperatures of the electrical activity of ion channels in the nerve membrane. Biochim Biophys Acta 602:610–620
Sanderman H (1978) Regulation of membrane enzymes by lipids. Biochim Biophys Acta 515:209–237
Satow Y, Kung C (1976) A “TEA-insensitive” mutant with increased potassium conductance inParamecium aurelia. J Exp Biol 65:51–63
Satow Y, Kung C (1979) Voltage-sensitive Ca-channels and the transient inward current inParamecium tetraurelia. J Exp Biol 78:149–161
Satow Y, Kung C (1981) Possible reduction of surface charge by a mutation inParamecium tetraurelia. J Membr Biol 59:179–190
Satow Y, Murphy AD, Kung C (1983) The ionic basis of the depolarization mechanoreceptor potential ofParamecium tetraurelia. J Exp Biol 103:253–264
Sonneborn TM (1970) Methods inParamecium research. In: Prescott D (ed) Methods in cell physiology, vol 4. Academic Press, New York, pp 241–339
Takahashi M, Naitoh Y (1978) Behavioral mutants ofParamecium caudatum with defective membrane electrogenesis. Nature 271:656–659
Toyotama H (1981) Thermo-receptor potential inParamecium. Ph D thesis, Osaka University, Japan
Whitaker BD, Poff KL (1980) Thermal adaptation of thermosensing and negative thermotaxis inDictyostelium. Exp Cell Res 128:87–93
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hennessey, T.M., Saimi, Y. & Kung, C. A heat-induced depolarization ofParamecium and its relationship to thermal avoidance behavior. J. Comp. Physiol. 153, 39–46 (1983). https://doi.org/10.1007/BF00610340
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00610340