Summary
The cells of origin of the trochlear nerve of urodeles, anurans and gymnophionans were labelled with HRP in order to compare the location and morphology of trochlear motoneurons and to find evidence for sensory fibers in the trochlear nerve of amphibians. Trochlear motoneuron perikarya were found in a ventral tegmental position predominantly on the contralateral side, but an ipsilateral cell was present in some specimens of urodeles and anurans. About 19 motoneurons were labelled inAmbystoma, about 60 motoneurons inXenopus, and a maximum of 7 cells inIchthyophis. Decussation of trochlear nerve fibers showed only inXenopus a highly variable pattern.
In urodeles, selective filling of the trochlear nerve labelled in addition to trochlear motoneurons a caudo-medial tectal group of about 20 neurons of the nucleus of the mesencephalic root of the trigeminal nerve. Gymnophionans showed also labelled cells of the mesencephalic trigeminal root in the caudal midbrain close to the trochlear nerve root. In some frogs, a few cells of the mesencephalic trigeminal root were labelled in the caudal tectum and occasionally in the velum medullare anterius.
Comparison of the numbers of trochlear nerve fibers with HRP-labelled motoneurons revealed inXenopus a proportion of 1.2:1, but of 2.7:1 inAmbystoma. However, counting both labelled motoneurons and cells of the mesencephalic trigeminal root resulted in a trochlear nerve fiber to labelled neuron proportion of 1.3:1 inAmbystoma much like inXenopus. The numbers of superior oblique muscle fibers and of trochlear nerve fibers, but not of HRP-labelled motoneurons, increased significantly with size inXenopus laevis. We suggest that increased peripheral branching of individual fibers within the trochlear nerve with size rather than differentiation of additional motoneurons takes place in growing postmetamorphicXenopus.
In contrast to other vertebrates studied so far, the trochlear nerve is a mixed nerve inAmbystoma and perhaps inIchthyophis. Whether this reflects a primitive or a derived condition is at present unclear.
Similar content being viewed by others
Abbreviations
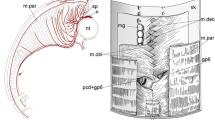
- ap :
-
alar plate
- av :
-
nucleus anteroventralis tegmenti
- bp :
-
basal plate
- flm :
-
fasciculus longitudinalis medialis
- ip :
-
nucleus interpeduncularis
- is :
-
nucleus isthmi
- it :
-
isthmic tegmentum
- nIV :
-
nervus trochlearis
- pd :
-
nucleus posterodorsalis tegmenti
- pr :
-
nucleus pretrigeminalis
- prm :
-
nucleus profundus mesencephali
- pv :
-
nucleus posteroventralis tegmenti
- rm :
-
nucleus reticularis medius
- rs :
-
nucleus reticularis superior
- si :
-
sulcus isthmi
- t :
-
teclum mesencephali
- tel :
-
telencephalon
- teg :
-
tegmentum mesencephali
- ts :
-
torus semicircularis
- vis :
-
n. visceralis secundarius
- III :
-
third cranial ventricle
- IV :
-
fourth cranial ventricle
References
Ariëns Kappers CU, Huber GC, Crosby EC (1936) The comparative anatomy of the nervous system of vertebrates, including man. Macmillan, New York
Craigmyle MBL (1985) The mixed cranial nerves. Wiley and Sons, Chichester
Daunicht WJ, Jaworski E, Eckmiller R (1985) Afferent innervation of extraocular mucles in the rat studied by retrograde and anterograde horseradish peroxidase transport. Neurosci Lett 56:143–148
Easter SS (1979) The growth and development of the superior oblique muscle and trochlear nerve in juvenile and adult goldfish. Anat Rec 195:683–698
Ebbesson SOE, Tang D (1965) A method for estimating the number of cells in histological sections. J R Micros Sco 84:449–464
Escher G, Schönenberger N, van der Loos H (1983) Detergent soaked HRP-chips: a new method for precise and effective delivery of small quantities of the tracer to nervous tissue. J Neurosci Methods 9:87–94
Floderus S (1944) Untersuchungen über den Bau der menschlichen Hypophyse mit besonderer Berücksichtigung der quantitativen morphologischen Verhältnisse. Acta Pathol Microbiol Scand 53:1–276
Finger TE, Rovainen CM (1978) Retrograde HRP labelling of the oculomotorneurons in adult lampreys. Brain Res 154:123–127
Francis ETR (1934) The anatomy of the salamander. Clarendon Press, Oxford
Fritzsch B, Nikundiwe AM (1984) Tracing neuronal connections in whole mounted brains of small vertebrates. Mikroskopie 41(1984):145–149
Fritzsch B, Nikundiwe AM, Will U (1984) Projection patterns of lateral-line afferents in anurans: A comparative HRP study. J Comp Neurol 229:451–469
Fritzsch B, Himstedt W, Crapon de Caprona MD (1985) Visual projections in larvalIchthyophis kohtaoensis (Amphibia: Gymnophiona). Dev Brain Res 23:201–210
Gacek RR (1974) Localization of neurons supplying the extraocular muscles in the kitten using horseradish peroxidase. Exp Neurol 44 (1974):381–403
Gomez Segade LA, Labandeira Garcia JL (1983) Location and quantitative analysis of the motoneurons innervating the extraocular muscles of the guinea pig, using horseradish peroxidase (HRP) and double or triple labelling with fluorescent substances. J Hirnforsch 24:613–626
Graf W, Brunken WJ (1984) Elasmobranch oculomotor organization: anatomical and theoretical aspects of the phylogenetic development of vestibulo-oculomotor connectivity. J Comp Neurol 227:569–581
Haller von Hallerstein V (1934) Kranialnerven. In: Bolk L, Göppert E, Kallius E, Lubosch W (eds) Handbuch der vergleichenden Anatomie der Wirbeltiere, vol 2. Urban und Schwarzenberg, Wien, pp 670–683
Herrick CJ (1948) The Brain of the Tiger Salamander. University of Chicago Press, Chicago
Hiscock J, Straznicky C (1982) Peripheral and central terminations of axons of the mesencephalic trigeminal neurons inXenopus. Neurosci Lett 32:235–240
Hiscock J, Straznicky C (1986) The formation of axonal projections of the mesencephalic trigeminal neurons in chick embryos. J Embryol Exp Morphol 93:281–290
Knöpfel T, Hess BJM, Precht W (1984) Responses of frog trochlear motoneurons to linear acceleration. J Comp Physiol A 154:233–240
Kollros JJ (1984) Growth and death of cells of the mesencephalic fifth nucleus inRana pipiens larvae. J Comp Neurol 224:386–394
Kuhlenbeck H (1974) The Central Nervous System of Vertebrates, vol IV. Karger, Basel
Labandeira Garcia JL, Gomez Segade LA, Suarez Nunez JM (1983) Localisation of motoneurons supplying the extra-ocular muscles of the rat using horseradish peroxidase and fluorescent double labelling. J Anat 137:247–261
Lang J, Reiter W (1986) Praktisch-ärztliche Befunde zum N. trochlearis. J Hirnforsch 27:101–110
Lowe DA, Russell IJ (1984) The relation between soma position and fibre trajectory of neurons in the mesencephalic trigeminal nucleus ofXenopus laevis. Proc R Soc Lond [Biol] 221:437–454
Luiten PGM (1979) Proprioceptive reflex connections of head musculature and the mesencephalic trigeminal nucleus in the carp. J Comp Neurol 183:903–912
Luiten PGM, Dijkstra-de Vlieger HP (1978) Extraocular muscle representation in the brainstem of the carp. J Comp Neurol 179:669–676
Maier A, DeSantis M, Eldred E (1974) The occurence of muscle spindles in extraocular muscles of various vertebrates. J Morphol 143:397–408
Matesz C, Székely G (1977) The dorsomedial group of cranial nerves in the frog. Acta Biol Acad Sci Hung 28:461–474
Miyazaki S (1985) Bilateral innervation of the superior oblique muscle by the trochlear nucleus. Brain Res 348:52–56
Narayanan GH, Narayanan YJ (1978) Determination of the embryonic origin of the mesencephlic nucleus of the trigeminal nerve in birds. J Embryol Exp Morphol 43:85–105
Naujoks-Manteuffel C, Manteuffel G, Himstedt W (1986) Localisation of motoneurons innervating the extraocular muscles inSalamandra salamandra L. (Amphibia, Urodela). J Comp Neurol 254:133–140
Nieuwenhuys R (1977) The brain of the lamprey in a comparative perspective. Ann New York Acad Sci 299:97–145
Nikundiwe AM, Nieuwenhuys R (1983) The cell masses in the brainstem of the South African clawed frogXenopus laevis: a topographical and topological analysis. J Comp Neurol 213:199–219
Opdam P, Nieuwenhuys R (1976) Topological analysis of the brain stem of the axolotlAmbystoma mexicanum. J Comp Neurol 165:285–306
Oppenheim R (1986) The absence of significant postnatal motoneuron death in the brachial and lumbar spinal cord of the rat. J Comp Neurol 246:281–286
Rosiles JR, Leonard RB (1980) The organization of the extraocular motor nuclei in the Atlantic stingray.Dasyatis sabina. J Comp Neurol 193:677–687
Ruskell GL (1984) Spiral nerve endings in human extraocular muscles terminate in motor end plates. J Anat 139:33–43
Scherer S, Easter SS (1984) Degenerative and regenerative changes in the trochlear nerve of goldfish. J Neurocytol 13:519–565
Scherer SS (1986) Reinnervation of the extraocular muscles in goldfish is nonselective. J Neurosci 6:765–773
Sohal GS, Holt RK (1978) Identification of the trochlear motoneurons by retrograde transport of horseradish peroxidase. Exp Neurol 59:509–514
Sohal GS, Weidman TA (1978) Development of the trochlear nerve: loss of axons during normal ontogeny. Brain Res 142:455–465
Sohal GS, Knox TS, Allen JC, Arumugam T, Campbell LR, Yamashita T (1985) Development of the trochlear nucleus in quail and comparative study of the trochlear nucleus, nerve, and innervation of the superior oblique muscle in quail, chick, and duck. J Comp Neurol 239:227–236
Sonntag R, Fritzsch B (1987) The development of the trochlear nucleus in amphibians: a quantitative HRP study. Neurosci Lett 77:143–148
Sperry DG, Grobstein P (1983) Postmetamorphic changes in the lumbar lateral motor column in relation to muscle growth in the toad,Bufo americanus. J Comp Neurol 216:104–114
Walberg F (1984) On the morphology of the mesencephalic trigeminal cells. New data based on tracer studies. Brain Res 322:119–123
Wake MH (1985) The comparative morphology and evolution of the eyes of caecilians (Amphibia, Gymnophiona). Zoomorph 105:277–295
Zaki W (1960) Le nerf pathétique chez l'homme. Etude relative à son origine, son trajet intracérébral et à sa structure. Arch Anat Histol Embryol 43:105–120
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fritzsch, B., Sonntag, R. The trochlear nerve of amphibians and its relation to proprioceptive fibers: a qualitative and quantitative HRP study. Anat Embryol 177, 105–114 (1987). https://doi.org/10.1007/BF00572534
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00572534