Abstract
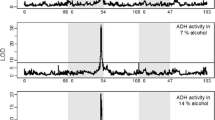
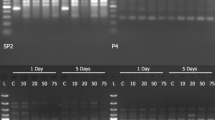
The effects of environmental 2-propanol on the in vivo properties of Drosophila alcohol dehydrogenase (E.C. 1.1.1.1.) are presented. Exposed flies were found to exhibit a significant decrease in ADH specific activity with a concomitant increase in the enzyme's relative in vivo stability and concentration. The possible adaptive significance of the observed responses is discussed.
Similar content being viewed by others
References
Ainsley, R., and Kitto, G. B. (1975). Selection mechanisms maintaining alcohol dehydrogenase polymorphisms in Drosophila melanogaster. In Markert, C. (ed.), Isozymes Vol. II, Academic, New York, p. 733.
Anderson, S. M., and McDonald, J. F. (1981). A method for determining the in vivo stability of Drosophila alcohol dehydrogenase (E.C.1.1.1.1.). Biochem. Genet. 19411.
Ayala, F. J., Powell, J. R., Tracy, M. L., Mourao, A., and Perez-Salas, S. (1972). Enzyme variability in the Drosophila willistoni group. IV. Genic variation in natural populations of Drosophila willistoni. Genetics 70113.
Davis, B. J., (1964). Disc electrophoresis II. Method and application to human serum proteins. Ann. N. Y. Acad. Sci. 121404.
Day, T. H., Hillier, P. C., and Clarke, B. (1974a). Properties of genetically polymorphic isozymes of alcohol dehydrogenase in Drosophila melanogaster. Biochem. Genet. 11141.
Day, T. H., Hillier, P. C., and Clarke, B. (1974b). The relative quantities and catalytic activities of enzymes produced by alleles at the alcohol dehydrogenase locus in Drosophila melanogaster. Biochem. Genet. 11155.
Day, T. H., and Needham, L. (1974). Properties of alcohol dehydrogenase isozymes in a strain of Drosophila melanogaster homozygous for the Adh-Slow allele. Biochem. Genet. 11167.
Everse, J., Zoll, E., Kahon, L., and Kaplan, N. (1971). Addition products of diphosphopyridine nucleotides with substrates of pyridine nucleotide-linked dehydrogenases. Biorg. Chem. 1207.
Fahey, J., and McKelvey, E. (1965). Quantitative determination of serum immunoglobulins in antibody-agar plates. J. Immunol. 9484.
Fletcher, T. S., Ayala, F. J., Thatcher, D. R., and Chambers, G. K. (1978). Structural analysis of the ADHS electromorph of Drosophila melanogaster. Proc. Natl. Acad. Sci USA 755609.
Fritz, R. F., and Pruitt, K. M. (1977). Intracellular turnover of isozymes. Isozymes: Current Topics Biol. Med. Res. 1125.
Gelfand, L. J., and McDonald, J. F. (1980). Relationship between ADH activity and behavioral response to environmental alcohol in Drosophila. Behav. Genet. 10237.
Gibson, J. (1970). Enzyme flexibility in D. melanogaster. Nature 227959.
Goldberg, G. L., and Dice, J. F. (1974). Intracellular protein degradation in mammalian and bacterial cells. Ann. Rev. Biochem. 43835.
Grell, E. H., Jacobson, B. K., and Murphy, J. B. (1968). Alterations of genetic material for analysis of alcohol dehydrogenase isozymes of Drosophila melanogaster. Ann. N. Y. Acad. Sci. 151441.
Heed, W. B. (1978). Ecology and genetics of Sonoran Desert Drosophila. In Brussard, P. F. (ed.), Ecological Genetics—the Interface Springer-Verlag, New York, p. 109.
Hewitt, N. E., Pipkin, S. B., Williams, N., and Chakrabartty, P. (1974). Variation in ADH activity in Class I and Class II strains of Drosophila. J. Hered. 65141.
Horikawa, M., Ling, L., and Fox, A. S. (1967). Effects of substrates on gene-controlled enzyme activities in cultured embryonic cells of Drosophila. Genetics 55569.
Jacobson, K. B. (1968). Alcohol dehydrogenase of Drosophila: interconversion of isozymes. Science 159324.
Jacobson, K. B., Murphy, J. B., Knopp, J. A., and Oritz, J. R. (1972). Multiple forms of Drosophila alcohol dehydrogenase. III. Conversion of one form to another by nicotinamide adenine dinucleotide or acetone. Arch. Biochem. Biophys. 14922.
Katunuma, N., Kominami, E., and Kominami, S. (1971a). A new enzyme that specifically inactivates apo-protein of pyridoxal enzymes. Biochem. Biophys. Res. Commun. 4570.
Katunuma, N., Kito, K., and Kominami, E. (1971b). A new protein that specifically inactivates apo-protein of NAD-dependent dehydrogenases. Biochem. Biophys. Res. Commun. 4576.
Litwack, G., and Rosenfield, S. (1973). Coenzyme dissociation, a possible determinant of short half-life inducible enzymes in mammalian liver. Biochem. Biophys. Res. Commun. 52181.
Mancini, G., Carbonara, G. O., and Heremans, J. F. (1965). Immunochemical quantification of antigens by single radial immunodiffusion. Immunochemistry 2235.
McDonald, J. F., and Avise, J. C. (1976). Evidence for the adaptive significance of enzyme activity levels: interspecific variation in α-GPDH and ADH in Drosophila. Biochem. Genet. 14347.
McDonald, J. F., Chambers, G., David, J., and Ayala, F. J. (1977). Adaptive response due to changes in gene regulation: a study with Drosophila. Proc. Natl. Acad. Sci. USA 744562.
McDonald, J. F., and Ayala, F. J. (1978). Genetic and biochemical basis of enzyme activity variation in natural populations I. Alcohol dehydrogenase in Drosophila melanogaster. Genetics 89371.
McDonald, J., Anderson, S., and Santos, M. (1980). Biochemical differences between products of the Adh-locus in Drosophila. Genetics 951013.
McReynolds, M. S., and Kitto, G. B. (1970). Purification and properties of Drosophila malate dehydrogenases. Biochim. Biophys. Acta 198165.
O'Donnell, J., Gerace, L., Leister, F., and Sofer, W. (1975). Chemical selection of mutants that affect alcohol dehydrogenase in Drosophila II. Use of 1-Pentyne-3-ol. Genetics 7973.
Ornstein, L. (1964). Disc electrophoresis I. Background and theory. Ann. N. Y. Acad. Sci. 121321.
Ouchterlony, O. (1953). Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol. Microbiol. Scand. 22231.
Papel, I., Henderson, M., Van Herrewege, J., David, J., and Sofer, W. (1979). Drosophila alcohol dehydrogenase: effects of exposing adults to acetone. Biochem. Genet. 17553.
Schwartz, M., and Sofer, W. (1976). Diet-induced alterations in distribution of multiple forms of alcohol dehydrogenase in Drosophila. Nature 263129.
Schwartz, M., O'Donnell, J., and Sofer, W. (1979). Origin of the multiple forms of alcohol dehydrogenase from Drosophila melanogaster. Arch. Biochem. Biophys. 194365.
Smith, I. (1968). Acrylamide gel disc electrophoresis. In Smith, I. (ed.), Chromatographic and Electrophoretic Techniques, Vol. II. Zone Electrophoresis. Wiley (Interscience), New York, p. 365.
Sofer, W. H., and Hatkoff, M. A. (1972). Chemical selection of alcohol dehydrogenase mutants in Drosophila. Genetics 72545.
Ursprung, H., and Leone, J., (1965). Alcohol dehydrogenase: a polymorphism in Drosophila melanogaster. J. Exp. Zool. 160147.
Vigue, C., and Johnson, F. (1973). Isozyme variability in species of the genus Drosophila VI. Frequency-property-environment relationships of allelic alcohol dehydrogenases in D. melanogaster. Biochem. Genet. 9213.
Author information
Authors and Affiliations
Additional information
This work was supported by NSF grant #DEB 7815466 to J.M. Journal Paper No. J-9979 of the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa. Project No. 2272.
Rights and permissions
About this article
Cite this article
Anderson, S.M., McDonald, J.F. Effect of environmental alcohol on in Vivo properties of Drosophila alcohol dehydrogenase. Biochem Genet 19, 421–430 (1981). https://doi.org/10.1007/BF00504285
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00504285