Abstract
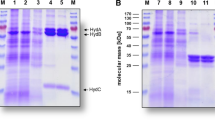
Hydrogenase from fructose-grown cells of Acetobacterium woodii has been purified 70-fold to a specific activity of 3,500 μmol hydrogen oxidized per min per mg of protein measured at 35°C and pH 7.6 with methyl viologen as electron acceptor. At the same conditions with reduced methyl viologen as electron donor the enzyme catalyzes the evolvement of 440 μmol of H2 per min per mg of protein. The enzyme was found in the soluble portion of the cell, indicating that it is either not membrane-bound or is loosely associated with the membrane. The purified enzyme, which does not contain nickel, exhibits spectroscopic properties similar to the iron-sulfur hydrogenase of Clostridium pasteurianum. The enzyme is strongly inhibited by carbon monoxide, with 50% inhibition occurring at approximately 7 nM CO. Ferredoxin, flavodoxin, and carbon monoxide dehydrogenase are reduced in hydrogen-dependent reaction by the A. woodi hydrogenase.
Similar content being viewed by others
Abbreviations
- CO dehydrogenase:
-
carbon monoxide dehydrogenase
- MV:
-
methyl viologen
- SDS:
-
sodium dodecyl sulfate
References
Adams MMW, Mortensen LE, Chen J-S (1981) Hydrogenase. Biochim Biophys Acta 594:105–176
Albracht SP, Graf EG, Thauer RK (1982) The EPR properties of nickel in hydrogenase from Methanobacterium thermoautotrophicum. FEBS Lett 140:311–313
Clark JE, Ragsdale SR, Ljungdahl LG, Wiegel J (1982) Levels of enzymes involved in the synthesis of acetate from CO2 in Clostridium thermoautotrophicum. J Bacteriol 151:507–509
Davis BJ (1964) Disc electrophoresis. II. Method and application to human serum proteins. Ann NY Acad Sci 121:404–427
Drake HL (1982) Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol 150:702–709
Drake HL, Hu S-I, Wood HG (1980) Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermoaceticum. J Biol Chem 255:7174–7180
Elliott JI, Brewer JM (1978) The inactivation of yeast enolase by 2,3-butanedione. Arch Biochem Biophys 190:351–357
Fernandez VM, Gutierrez C, Ballesteros A (1982) Determination of hydrogenase activity using an anaerobic spectrophotometric device. Anal Biochem 120:85–90
Graf E-G, Thauer RK (1981) Hydrogenase from Methanobacterium thermoautotrophicum a nickel-containing enzyme. FEBS Lett 136:165–167
Hedrick JL, Smith AJ (1968) Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys 126:155–164
Jones JB Jr (1977) Elemental analysis of soil extracts and plant tissue by plasma emission spectroscopy. Commun Soil Sci Plant Anal 8:349–365
Kojima N, Fox JA, Hausinger RP, Daniels L, Orme-Johnson WH, Walsh C (1983) Paramagnetic centers in the nickel-containing, deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum. Proc Natl Acad Sci USA 80:378–382
Krüger H-J, Huynh BH, Ljungdahl PO, Xavier AV, DerVartanian DV, Moura I, Peck HD Jr, Teixeira M, Moura JJG, LeGall J (1982) Evidence for nickel and a three-iron center in the hydrogenase of Desulfovibrio desulfuricans. J Biol Chem 257:14620–14623
Ljungdahl LG (1983) Formation of acetate using homoacetate fermenting anaerobic bacteria. In: Wise DL (ed) Organic chemicals from Biomass. Benjamin/Cummings Publ Comp, Menlo Park, CA, pp 219–248
Ljungdahl LG, Wood HG (1982) Acetate biosynthesis. In: Dolphin D (ed) B12, vol 2. John Wiley and Sons, New York, pp 166–202
Ljungdahl LG, O'Brien WE, Moore MR, Liu M-T (1980) Methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum and methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate cyclohydrolase (combined) from Clostridium thermoaceticum. Methods Enzymol 66:599–609
Martin DR, Lundie LL, Kellum R, Drake HL (1983) Carbon monoxide-dependent evolution of hydrogen by the homoacetate-fermenting bacterium Clostridium thermoaceticum. Curr Microbiol 8:337–340
Peck HD Jr, Gest HA (1956) A new procedure for assay of bacterial hydrogenases. J Bacteriol 71:70–80
Pezacka E, Wood HG (1984) The synthesis of acetyl-CoA by Clostridium thermoaceticum from carbon dioxide, hydrogen, coenzyme A and methyltetrahydrofolate. Arch Microbiol 137:63–69
Rabinowitz JC (1975) Pyruvate-ferredoxin oxidoreductase from Clostridium acidi-urici. Methods Enzymol 41:334–337
Ragsdale SW, Clark JE, Ljungdahl LG, Lundie LL, Drake HL (1983a) Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem 258:2364–2369
Ragsdale SW, Ljungdahl LG, DerVartanian DV (1983b). Isolation of carbon monoxide dehydrogenase from Acetobacterium woodii and comparison of its properties with those of the Clostridium thermoaceticum enzyme. J Bacteriol 155:1224–1237
Ragsdale SW, Ljungdahl LG (1984) Characterization of ferredoxin, flavodoxin, and rubredoxin from Clostridium formicoaceticum grown in media with high and low iron contents. J Bacteriol 157:1–6
Reisner AH, Nemes P, Bucholtz C (1975) The use of Coomassie brilliant blue G-250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels/ Anal Biochem 64:509–516
Weber K, Pringle JR, Osborn M (1972) Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol 26:3–27
Yamamoto I, Saiki T, Liu S-M, Ljungdahl LG (1983) Purification and properties of NADH-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem 258:1826–1832
Author information
Authors and Affiliations
Additional information
This paper is dedicated to Professor Dr. Hans G. Schlegel on the occasion of his 60-years birthday. Hans' contributions to the field of microbiology are many and it is a pleasure for us to commemorate him in this way. One of us, L. G. L., had the fortune as a Humboldt-Preis recipient to spend a year at the Institut für Mikrobiologie der Universität Göttingen. Besides the best possible working conditions memories involve pleasant evenings with a glass of Rhine-wine in the Schlegels' home to strenuous back-packing in the Austrian Alps.
Rights and permissions
About this article
Cite this article
Ragsdale, S.W., Ljungdahl, L.G. Hydrogenase from Acetobacterium woodii . Arch. Microbiol. 139, 361–365 (1984). https://doi.org/10.1007/BF00408380
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00408380