Abstract
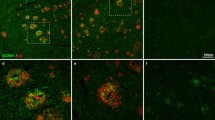
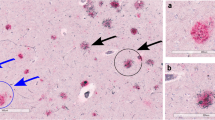
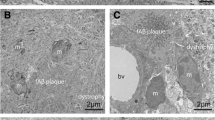
The incorporation of neurites into amyloid deposits is an important step in the formation of senile plaques in Alzheimer's disease. It is unknown whether all neuronal types contribute neurites equally to plaques, or whether the processes of certain types are preferentially incorporated. We addressed this question by comparing the incorporation into neocortical plaques of neurites containing the widely distributed neuronal markers chromogranin A (CgA), parvalbumin (PV) and calbindin D-28K (CaBP) in relation to the number of neuronal perikarya expressing each of these substances in the neocortex. We found a consistent, statistically significant ranking, so that CgA-immunoreactive (ir) neurites were preferentially incorporated into plaques in comparison with PV-ir, and PV-ir were favoured over CaBP-ir neurites.
Similar content being viewed by others
References
Adams LA, Ang LC, Munoz DG (1993) Chromogranin A, a soluble synaptic vesicle protein, is found in cortical neurons other than previously identified peptidergic neurons in the human neocortex. Brain Res 602:336–341
Armstrong DM, Benzing WC, Evans J, Terry RD, Shields D, Hansen LA (1989) Substance P and somatostatin coexist within neuritic plaques: implications for the pathogenesis of Alzheimer's disease. Neuroscience 31:663–671
Baimbridge KG, Miller JJ (1982) Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res 245:223–229
Braak H, Braak E, Kalus P (1989) Alzheimer's disease: areal and laminar pathology in the occipital isocortex. Acta neuropathol 77:494–506
Celio MR, Baier W, Scharer L, de Viragh PA, Gerday C (1988) Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium 9:81–86
Chang HT, Kuo H (1991) Relationship of calbindin D-28k and cholinergic neurons in the nucleus basalis of Meynert of the monkey and the rat. Brain Res 549:141–145
Conover WJ (1980) Practical Nonparametric Statistics. Wiley, Toronto
DeFelipe J, Jones EG (1991) Parvalbumin immunoreactivity reveals layer IV of monkey cerebral cortex as a mosaic of microzones of thalamic afferent terminations. Brain Res 562:39–47
DeFelipe J, Hendry SH, Jones EG (1989) Visualization of chandelier cell axons by parvalbumin immunoreactivity in monkey cerebral cortex. Proc Natl Acad Sci USA 86:2093–2097
DeFelipe J, Hendry SH, Jones EG (1989) Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res 503:49–54
Galindo E, Rill A, Bader MF, Aunis D (1991) Chromostatin, a 20-amino acid peptide derived from chromogranin A, inhibits chromaffin cell secretion. Proc Natl Acad Sci USA 88:1426–1430
Giaccone G, Tagliavini F, Linoli G, Bouras C, Frigerio L, Frangione B, Bugiani O (1989) Down patients: extracellular preamyloid deposits precede neuritic degeneration and senile plaques. Neurosci Lett 97:232–238
Hendry SH, Jones EG, Emson PC, Lawson DE, Heizmann CW, Streit P (1989) Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res 76:467–472
Herzig KH, Louie DS, Tatemoto K, Chung OY (1992) Pancreastatin inhibits pancreatic enzyme secretion by presynaptic modulation of acetylcholine release. Am J Physiol 262:G113-G117
Hof PR, Morrison JH (1991) Neocortical neuronal subpopulations labeled by a monoclonal antibody to calbindin exhibit differential vulnerability in Alzheimer's disease. Exp Neurol 111:293–301
Hof PR, Cox K, Young WG, Celio MR, Rogers J, Morrison JH (1991) Parvalbumin-immunoreactive neurons in the neocortex are resistant to degeneration in Alzheimer's disease. J Neuropathol Exp Neurol 50:451–462
Hoffman SR, Kowall NW, McKee AC (1988) Calbindin D28 neurons in the hippocampal formation are resistant to degeneration and do not develop regenerative features in Alzheimer's Disease. Abstr Soc Neurosci 14:154
Ichimiya Y, Emson PC, Mountjoy CQ, Lawson DE, Heizmann CW (1988) Loss of calbindin-28K immunoreactive neurons from the cortex in Alzheimer-type dementia. Brain Res 475:156–159
Jones EJ, Hendry SHC (1989) Differential calcium binding protein immunoreactivity distinguishes classes of relay neurons in monkey thalamic nuclei. Eur J Neurosci 1:222–246
Kar S, Bretherton-Watt D, Gibson SJ et al (1989) Novel peptide pancreastatin: its occurrence and codistribution with chromogranin A in the central nervous system of the pig. J Comp Neurol 288:627–639
Kitt CA, Price DL, Struble RG, Cork LC, Wainer BH, Becher MW, Mobley WC (1984) Evidence for cholinergic neurites in senile plaques. Science 226:1443–1444
Kitt CA, Struble RG, Cork LC, Mobley WC, Walker LC, Joh TH, Price DL (1985) Catecholaminergic neurites in senile plaques in prefrontal cortex of aged non-human primates. Neuroscience 16:691–699
Kowall NW, Beal MF (1988) Cortical somatostatin, neuropeptide Y, and NADPH diaphorase neurons: normal anatomy and alterations in Alzheimer's disease. Ann Neurol 23:105–114
Lassmann H, Weiler R, Fischer P, Bancher C, Jellinger K, Floor E, Danielczyk W, Seitelberger F, Winkler H (1992) Synaptic pathology in Alzheimer's disease: immunological data for markers for synaptic and large dense-core vesicles. Neuroscience 46:1–8
Levey AI, Bolam JP, Rye DB, Hallanger AE, Dermuth RM, Mesulam MM, Wainer BH (1986) A light and electron microscopic procedure for sequential double antigen localization using diaminobenzidine and benzidine dihydrochloride. J Histochem Cytochem 34:1449–1457
Lewis DA, Lund JS (1990) Heterogeneity of chandelier neurons in monkey neocortex: corticotropin-releasing factor-and parvalbumin-immunoreactive populations. J Comp Neurol 293:599–615
McGuire BA, Hornung J-P, Gilbert CD, Wiesel TN (1984) Patterns of synaptic input to layer 4 of cat striate cortex. J Neurosci 4:3021–3033
McKee AC, Kosik KS, Kowall NW (1991) Neuritic pathology and dementia in Alzheimer's disease. Ann Neurol 30:156–165
Morrison JH, Rogers J, Scherr S, Benoit R, Bloom FE (1985) Somatostatin immunoreactivity in neuritic plaques of Alzheimer's patients. Nature 314:90–92
Munoz DG (1990) The distribution of chromogranin A-like immunoreactivity in the human hippocampus coincides with the pattern of resistance to epilepsy-induced neuronal damage. Ann Neurol 27:266–275
Munoz DG (1991) Chromogranin A-like immunoreactive neurites are major constituents of senile plaques. Lab Invest 64:826–832
Munoz DG, Wang D (1992) Tangle-associated neuritic clusters. A new lesion in Alzheimer's disease and aging suggests that aggregates of dystrophic neurites are not necessarily associated with beta/A4. Am J Pathol 140:1167–1178
Munoz DG, Kobylinski L, Henry DD, George DH (1990) Chromogranin A-like immunoreactivity in the human brain: distribution in bulbar medulla and cerebral cortex. Neuroscience 34:533–543
Nakamura S, Vincent SR (1986) Somatostatin and neuropeptide Y-immunoreactive neurons in the neocortex in senile dementia of Alzheimer's type. Brain Res 370:11–20
Probst A, Basler V, Bron B, Ulrich J (1983) Neuritic plaques in senile dementia of Alzheimer type: a Golgi analysis in the hippocampal region. Brain Res 268:249–254
Reiffen FU, Gratzl M (1986) Chromogranins, widespread in endocrine and nervous tissue, bind Ca2+. FEBS Lett 195:327–330
Rogers J, Morrison JM (1985) Quantitative morphology and regional and laminar distributions of senile plaques in Alzheimer's disease. J Neurosci 5:2801–2808
Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, Hockey A, Montgomery P, Beyreuther K, Masters CL (1989) Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease [see comments]. N Engl J Med 320:1446–1452
Selkoe DJ, Abraham CR, Podlisny MB, Duffy LK (1986) Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem 46:1820–1834
Seress L, Gulyas AI, Freund TF (1991) Parvalbumin- and calbindin D28k-immunoreactive neurons in the hippocampal formation of the macaque monkey. J Comp Neurol 313:162–177
Simon J-P, Bader M-F, Aunis D (1988) Secretion from chromaffin cells is controlled by chromogranin A-derived peptides. Proc Natl Acad Sci USA 85:1712–1716
Somogyi P, Takagi H (1982) A note on the use of picric acid-paraformaldehyde-glutaraldehyde fixative for correlated light and electron microscopic immunocytochemistry. Neuroscience 7:1779–1783
Somogyi P, Hodgson AJ, DePotter RW, Fischer-Colbrie R, Schober M, Winkler H, Chubb IW (1984) Chromogranin immunoreactivity in the central nervous syste. Immunochemical characterisation, distribution and relationship to catecholamine and enkephalin pathways. Brain Res 8:193–230
Struble RG, Powers RE, Casanova MF, Kitt CA, Brown EC, Price DL (1987) Neuropeptidergic systems in plaques of Alzheimer's disease. J Neuropathol Exp Neurol 46:567–584
Verga L, Frangione B, Tagliavini F, Giaccone G, Migheli A, Bugiani O (1989) Alzheimer patients and Down patients: cerbral preamyloid deposits differ ultrastructurally and histochemically from the amyloid of senile plaques. Neurosci Lett 105:294–299
Walker LC, Kitt CA, Struble RG, Schmechel DE, Oertel WH, Cork LC, Price DL (1985) Glutamic acid decarboxylase-like immunoreactive neurites in senile plaques. Neurosci Lett 59:165–169
Weiler R, Lassmann H, Fischer P, Jellinger K, Winkler H (1990) A high ratio of chromogranin A to synaptin/synaptophysin is a common feature of brains in Alzheimer and Pick disease. FEBS Lett 263:337–339
Wilson BS, Lloyd RV (1984) Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol 115:458–468
Yamaguchi H, Nakazato Y, Hirai S, Shoji M, Harigaya Y (1989) Electron micrograph of diffuse plaques. Initial stage of senile plaque formation in the Alzheimer brain. Am J Pathol 135:593–597
Yamaguchi H, Nakazato Y, Shoji M, Takatama M, Hirai S (1991) Ultrastructure of diffuse plaques in senile dementia of the Alzheimer type: comparison with primitive plaques. Acta Neuropathol 82:13–20
Yoo SH, Albanesi JP (1991) High capacity, low affinity Ca2+ binding of chromogranin A. Relationship between the pH-induced conformational change and Ca2+ binding property. J Biol Chem 266:7740–7745
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Adams, L.A., Munoz, D.G. Differential incorporation of processes derived from different classes of neurons into senile plaques in Alzheimer's disease. Acta Neuropathol 86, 365–370 (1993). https://doi.org/10.1007/BF00369449
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00369449