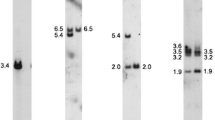
Abstract
The asymmetric unit membrane (AUM) of the apical surface of mammalian urinary bladder epithelium contains several major integral membrane proteins, including uroplakins IA and IB (both 27 kDa), II (15 kDa), and III (47 kDa). These proteins are synthesized only in terminally differentiated bladder epithelial cells. They are encoded by separate genes and, except for uroplakins IA and IB, appear to be unrelated in their amino acid sequences. The genes encoding these uroplakins were mapped to chromosomes of cattle through their segregation in a panel of bovine x rodent somatic cell hybrids. Genes for uroplakins IA, IB, and II were mapped to bovine (BTA) Chromosomes (Chrs) 18 (UPK1A), 1 (UPK1B), and 15 (UPK2), respectively. Two bovine genomic DNA sequences reactive with a uroplakin III cDNA probe were identified and mapped to BTA 6 (UPK3A) and 5 (UPK3B). We have also mapped genes for uroplakins 1A and II in mice, to the proximal regions of mouse Chr 7 (Upk1a) and 9 (Upk2), respectively, by analyzing the inheritance of restriction fragment length variants in recombinant inbred mouse strains. These assignments are consistent with linkage relationships known to be conserved between cattle and mice. The mouse genes for uroplakins IB and III were not mapped because the mouse genomic DNA fragments reactive with each probe were invariant among the inbred strains tested. Although the stoichiometry of AUM proteins is nearly constant, the fact that the uroplakin genes are unlinked indicates that their expression must be independently regulated. Our results also suggest likely positions for two human uroplakin genes and should facilitate further analysis of their possible involvement in disease.
Similar content being viewed by others
References
Alroy, J., Merk, F.B., Moire, J., Weinstein, R.S. (1982). Membrane differentiation in the Golgi apparatus of mammalian urinary bladder epithelium. Anat. Rec. 203, 429–440.
Blank, R.D., Campbell, G.R., Calabro, A., D'Eustachio, P. (1988). A linkage map of mouse Chromosome 12: localization of Igh and effects of sex and interference on recombination. Genetics 120, 1073–1083.
D'Eustachio, P., Jadidi, S., Fuhlbrigge, R.C., Gray, P.W., Chaplin, D.D. (1987). Interleukin-1 α and β genes: linkage on chromosome 2 in the mouse. Immunogenetics 26, 339–343.
Hicks, R.M. (1965). The fine structure of the transitional epithelium of the rat ureter. J. Cell Biol. 26, 25–48.
Hicks, R.M. (1966). The permeability of rat transitional epithelium: keratinization and the barrier to water. J. Cell Biol. 28, 21–31.
Hicks, R.M. (1975). The mammalian urinary bladder: an accommodating organ. Biol. Rev. 50, 215–246.
Hotta, H., Ross, A.H., Huebner, K., Isobe, M., Wendeborn, S., Chao, M.V., Ricciardi, R.P., Tsujimoto, Y., Croce, C.M., Koprowski, H. (1988). Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res. 48, 2955–2962.
Kallin, B., de Martin, R., Etzold, T., Sorrentino, V., Philipson, L. (1991). Cloning of a growth arrest-specific and transforming growth factor β-regulated gene, TI 1, from an epithelial cell line. Mol. Cell. Biol. 11, 5338–5345.
Lewis, S.A., deMoura, J.L.C. (1984). Apical membrane area of rabbit urinary bladder increases by fusion of intracellular vesicles: an electrophysiological study. J. Membrane Biol. 82, 123–136.
Lin, J.-H., Wu, X.R., Kreibich, G., Sun, T.-T. (1993). Precursor sequence, processing, and urothelial specific expression of a major 15-KD protein subunit of asymmetric unit membrane. J. Biol. Chem., in press.
Marken, J.S., Schiever, G.L., Hellström, I., Hellström, K.E., Aruffo, A. (1992). Cloning and expression of the tumor-associated antigen L6. Proc. Natl. Acad. Sci. USA 89, 3503–3507.
Minsky, B.D., Chlapowski, F.J. (1978). Morphometric analysis of the translocation of transitional epithelial cells during the expansion-contraction cycles of mammalian urinary bladder. J. Cell Biol. 77, 685–697.
O'Brien, S.J., Womack, J.E., Lyons, L.A., Moore, K.J., Jenkins, N.A., Copeland, N.G. (1993). Anchored reference loci for comparative genome mapping in mammals. Nature Genet. 3, 103–112.
Oren, R., Takahashi, S., Doss, C., Levy, R., Levy, S. (1990). TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol. Cell. Biol. 10, 4007–4015.
Porter, K.R., Bonneville, M.A. (1963). An Introduction to the Fine Structure of Cells and Tissues (New York, N.Y.: Lea & Febiger).
Ryan, A.M., Gallagher, D.S., Womack, J.E. (1992). Syntenic mapping and chromosomal localization of bovine α and β interferon genes. Mammalian Genome 3, 575–578.
Sant, G.R. (1991). Interstitial cystitis. Monographs Urol. 12, 37–63.
Silver, J. (1985). Confidence limits for estimates of gene linkage based on analysis of recombinant inbred strains. J. Hered. 76, 436–440.
Staehelin, L.A., Chlapowski, F.J., Bonneville, M.A. (1972). Luminal plasma membrane of the urinary bladder. I. Three dimensional reconstruction from freeze-etch images. J. Cell Biol. 53, 73–91.
Taylor, B.A. (1978). Recombinant inbred strains. In: Origins of Inbred Mice, H.C. Morse, III, ed. (New York, N.Y.: Academic Press), pp. 423–438.
Taylor, B.A. (1989). Recombinant inbred strains. In Genetic Variants and Strains of the Laboratory Mouse, M.F. Lyon, A.G. Searle, eds. (New York, N.Y.: Oxford University Press), pp. 773–796.
Womack, J.E. (1990). Gene mapping in the cow. In Domestic Animal Cytogenetics, Vol. 34, (San Diego, Calif.: Academic Press), pp. 251–271.
Womack, J.E., Moll, Y.D. (1986). Gene map of the cow: conservation with mouse and man. J. Hered. 77, 2–7.
Wright, M.D., Henkle, K.J., Mitchell, G.F. (1990). An immunologic Mr 23,000 integral membrane protein of Schistosoma mansoni worms that closely resembles a human tumor-associated antigen. J. Immunol. 144, 3195–3200.
Wu, X.R., Sun, T.-T. (1993). Molecular cloning of a 47-kDa tissue-specific and differentiation-dependent urothelial cell surface glycoprotein. J. Cell Sci., in press.
Wu, X.R., Manabe, M., Yu, J., Sun, T.-T. (1990). Large scale purification and immunolocalization of bovine uroplakins I, II and III. J. Biol. Chem. 265, 19170–19179.
Yu, J., Manabe, M., Wu, X.R., Xu, C., Surya, B., Sun, T.-T. (1990). Uroplakin I: a 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J. Cell Biol. 111, 1207–1216.
Yu, J., Lin, J.-H., Wu, X.R., Sun, T.-T. (1993). Existence of at least two forms of uroplakin I: the cloning and characterization of their cDNAs. Submitted.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ryan, A.M., Womack, J.E., Yu, J. et al. Chromosomal localization of uroplakin genes of cattle and mice. Mammalian Genome 4, 656–661 (1993). https://doi.org/10.1007/BF00360903
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00360903