Abstract
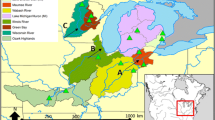
Species endemic to deep-sea hydrothermal vent ecosystems have disjunct distributions imposed by the island-like arrangement of their specialized habitats. Using allozyme electrophoresis, we examined genetic population structure of the hydrothermal vent amphipod Ventiella sulfuris Barnard and Ingram, 1990. Samples from five sites along the East Pacific Rise (EPR) and two along the Galapagos Rift were collected in 1990 and 1988, respectively. Variability, based on 12 enzyme loci, was relatively high \(\bar P\) (proportion of polymorphic loci whose most common allele not greater than 0.95 in frequency) =41.6%; \(\bar H\) (mean heterozygosity) =0.158] compared with shallow-water marine and freshwater amphipods, and similar to the deep-sea lysianassid Eurythenes gryllus. Genetic divergence among populations spread along a cöntiguous rift axis (i.e., EPR) was low [Nei's genetic distance (D) ranged from <0.001 to 0.018]. Genetic tructure analysis suggests that along a contiguous ridge axis migration occurs in a stepping stone manner and is unconstrained by distances as great as 1200 km (migration rate, \(\bar M\), ranged from 1.9 to 67.8 ind. generation−1). However, genetic divergence between populations on disjunct ridge axes was extremely high (D ranged from 0.438 to 0.476). Most of the variance in gene frequencies was due to the differences between the major subpopulations inhabiting the two distinct ridge axes, EPR and Galapagos Rift. Apparently, very little migration and gene flow occur between these major subpopulations (M≪1). This level of genetic divergence may be sufficient to justity separation of EPR and Galapagos Rift populations at the species level. Futher analyses of morphological characters is required before taxonomic status can be assigned.
Similar content being viewed by others
Literature cited
Ayala, F. J., Tracey, M. L., Barr, L. G., McDonald, J. F., Perez-Salas, S. (1974). Genetic variation in natural populations of five Drosophila species and the hypothesis of the selective neutrality of protein polymorphisms. Genetics 77: 343–384
Ayala, F. J., Valentine, J. W. (1974). Genetic variability in the cosmopolitan deep-water ophiuran Ophiomusium lymani. Mar. Biol. 27: 51–57
Baldwin, R. J., Smith, K. L., Jr. (1987). Temporal variation in the catch rate, length, color and sex of the necrophagus amphipod, Eurythenes gryllus, from the central and eastern north Pacific. Deep-Sea Res. 34: 425–439
Barnard, J. L. (1961). Gammaridean Amphipoda from depths of 400 to 6000 meters. Galathea Rep. 5: 23–128
Barnard, J. L., Ingram, C. (1990). Lysianassoid Amphipoda (Crustacea) from deep-sea thermal vents. Smithson. Contr. Zool. 499: 1–80
Bisol, P. M., Costa, R., Sibuet, M. (1984). Ecological and genetical survey on two deep-sea holothurians: Benthogone rosea and Benthodytes typica. Mar. Ecol. Prog. Ser. 15: 275–281
Blot, M., Soyer, J., Thiriot-Quievreux, C. (1987). Preliminary data on the genetic differentiation of Mytilus desolationis Lamy 1936 and Aulacomya ater regia Powell 1957 (Bivalvia, Mytilidae) in the Kerguelen Islands (Terres Australes et Antarctiques Françaises). Polar Biol. 7: 1–9
Bucklin, A. (1988). Allozymic variability of Riftia pachyptila populations from the Galapagos Rift and 21°N hydrothermal vents. Deep-Sea Res. 35: 1759–1768
Bucklin, A., Wilson, R. R., Jr., Smith, K. L., Jr. (1987). Genetic differentiation of seamount and basin populations of the deepsea amphipod Eurythenes gryllus. Deep-Sea Res. 34: 1795–1810
Bulnheim, H. P., Scholl, A. (1986). Genetic differentiation between populations of Talitrus saltator and Talorchestia deshayesii (Crustacea: Amphipoda) from coastal areas of the north-western European continent. Mar. Biol. 92: 525–536
Cary, S. C., Felbeck, H., Holland, N. D. (1989). Observations on the reproductive biology of the hydrothermal vent tube worm Riftia pachyptila. Mar. Ecol. Prog. Ser. 52: 89–94
Cavalli-Sforza, L. L., Edwards, A. W. F. (1967). Phylogenetic analysis: models and estimation procedures. Evolution, Lawrence, Kansas 21: 550–570
Childress, J. J. (1988). Hydrothermal vents: a case study of the biology and chemistry of a deep-sea hydrothermal vent of the Galapagos Rift. Deep-Sea Res. 35: 1677–1849
Costa, R., Bisol, P. M. (1978). Genetic variability in deep-sea organisms. Biol. Bull. mar. biol. Lab., Woods Hole 155: 125–133
Farris, J. F. (1981). Distance data in phylogenetic analysis. In: Funk, V. A., Brooks, D. R. (eds.) Advances in cladistics: proceedings of the first meeting of the Willi Hennig Society. New York Botanical Garden, New York, p. 1–23
Fornari, D. J., Gallo, D. G., Edwards, M. H., Madsen, J. A., Perfit, M. R., Shor, A. N. (1989). Structure and topography of the Siqueros transform fault system: evidence for the development of intra-transform spreading centers. Mar. geophys. Res. 11: 263–299
Gooch, J. L., Schopf, T. J. M. (1972). Genetic variability in the deep sea: relation to environmental variability. Evolution, Lawrence, Kansas 26: 545–552
Grassle, J. P. (1985). Genetic differentiation in populations of hydrothermal vent mussels (Bathymodiolus thermophilus) from the Galapagos Rift and 13°N on the East Pacific Rise. In: Jones, M. L. (ed.) Hydrothermal vents of the Eastern Pacific: an overview, Vol. 6. Bull. Biol. Soc. Wash, Washington D. C., p. 429–442
Grassle, J. F. (1986). The ecology of deep-sea hydrothermal vent communities. Adv. mar. Biol. 23: 301–362
Gustafson, R. G., Littlewood, D. T. J., Lutz, R. A. (1991). Gastropod egg capsules and their contents from deep-sea hydrothermal vent environments. Biol. Bull. mar. biol. Lab., Woods Hole 180: 34–55
Hessler, R. R., Lonsdale, P. F. (1991). Biogeography of Mariana Trough hydrothermal vent communities. Deep-Sea Res. 38: 185–199
Hessler, R. R., Smithey, W. M., Boudrias, M. A., Keller, C. H., Lutz, R. A., Childress, J. J. (1988). Temporal change in megafauna at the Rose Garden hydrothermal vent (Galapagos Rift; eastern tropical Pacific). Deep-Sea Res. 35: 1681–1709
Jones, M. L. (ed.) (1985). Hydrothermal vents of the Eastern Pacific: an overview, Vol. 6. Bull. Biol. Soc. Wash., Washington D. C., p. 1–566
Kimura, M., Weiss, W. H. (1964). The stepping stone model of genetic structure and the decrease of genetic correlation with distance. Genetics 49: 561–576
Kwast, K. E., Foltz, D. W., Stickle, W. B. (1990). Population genetics and systematics of the Leptasterias hexactis (Echinodermata: Asteroidea) species complex. Mar. Biol. 105: 477–489
Levene, H. (1949). On a matching problem arising in genetics. Ann. Math. Statist. 20: 91–94
Lonsdale, P. (1976). Abyssal circulation of the southwestern Pacific and some geological implications. J. geophys. Res. 81: 1163–1176
Lonsdale, P. (1988). Structural pattern of the Galapagos Microplate and evolution of the Galapagos Triple Junction. J. Geophys. Res. 93: 13551–13574
Lutz, R. A. (1988). Dispersal of organisms at deep-sea hydrothermal vents: a review. Oceanol. Acta (Spec. Vol.) 8: 23–29
Lutz, R. A., Jablonski, D., Rhoads, D. C., Turner, R. D. (1980). Larval dispersal of a deep-sea hydrothermal vent bivalve from the Galapagos Rift. Mar. Biol. 57: 127–133
Lutz, R. A., Jablonski, D., Turner, R. D. (1984). Larval development and dispersal at deep-sea hydrothermal vents. Science, N.Y. 226: 1451–1454
Mallet, A. L., Zouros, E., Gartner-Kepkay, K. E., Freeman, K. R., Dickie, L. M. (1985). Larval viability and heterozygote deficiency in populations of marine bivalves: evidence from pair matings of mussels. Mar. Biol. 87: 165–172
Murphy, L. S., Rowe, G. T., Haedrich, R. L. (1976). Genetic variability in deep-sea echinoderms. Deep-Sea Res. 23: 339–348
Murphy, R. W., Sites, J. W., Jr., Buth, D. G., Haufler, C. H. (1990). Proteins I: isozyme electrophoresis. In: Hillis, D. M., Moritz, C. (eds.) Molecular systematics. Sinauer Associates, Inc., Sunderland, Massachusetts, p. 45–126
Nei, M. (1972). Genetic distance between populations. Am. Nat. 106: 283–292
Nei, M. (1973). Analysis of gene diversity in subdivided populations. Proc. Natn. Acad. Sci. U.S.A. 70: 3321–3323
Nei, M. (1977). F-statistics and analysis of gene diversity in subdivided populations. Ann. Hum. Genet. 41: 225–233
Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89: 583–590
Nei, M. (1987). Molecular evolutionary genetics. Columbia University Press, New York
Patarnello, T., Bisol, P. M., Varotto, V., Fuser, V., Battaglia, B. (1990). A study of enzyme polymorphism in the antarctic amphipod Paramoera walkeri Stebbing. Polar Biol. 10: 495–498
Porter, A. H. (1990). Testing nominal species boundaries using gene flow statistics: the taxonomy of two hybridizing adminal butterflies (Limenitis: Nymphalidae). Syst. Zool. 39: 131–147
Rice, W. R. (1989). Analyzing tables of statistical tests. Evolution, Lawrence, Kansas 43: 223–225
Scheepmaker, M. (1990). Genetic differentiation and estimated levels of gene flow in members of the Gammarus pulex-group (Crustacea, Amphipoda) in western Europe. Bijdr. Dierk. 60: 3–30
Siebenaller, J. F. (1978). Genetic variation in deep-sea invertebrate populations: the bathyal gastropod Bathybembix bairdii. Mar. Biol. 47: 265–275
Siegismund, H. R., Simonsen, V., Kolding, S. (1985). Genetic studies of Gammarus. I. Genetic differentiation of local populations. Hereditas 102: 1–13
Slatkin, M., Barton, N. H. (1989). A comparison of three indirect methods for estimating average levels of gene flow. Evolution Lawrence, Kansas 43: 1349–1368
Slatkin, M., Maddison, W. P. (1990). Detecting isolation by distance using phylogenies of genes. Genetics 126: 249–260
Slatkin, M., Voelm, L. (1991). F ST in a hierarchical island model. Genetics 127: 627–629
Swofford, D. L. (1981). On the utility of the distance Wagner procedure. In: Funk, V. A., Brooks, D. R. (eds.) Advances in cladistics: proceedings of the first meeting of the Willi Hennig Society. New York Botanical Garden, New York, p. 25–43
Swofford, D. L., Selander, R. B. (1989). BIOSYS-1: a computer program for the analysis of allelic variation in population genetics and biochemical systematics, release 1.7. David L. Swofford, Illinois Natural History Survey, Champaign, Illinois
Thurston, M. H. (1990). Abyssal necrophagus amphipods (Crustacea: Amphipoda) in the northeast and tropical Atlantic Ocean. Prog. Oceanogr. 24: 257–274
Tracey, M. L., Nelson, K., Hedgecock, D., Shleser, R. A., Pressick, M. L. (1975). Biochemical genetics of lobsters: genetic variation and the structure of American lobster (Homarus americanus) populations. J. Fish. Res. Bd Can. 32: 2091–2101
Tunnicliffe, V. (1988). Biogeography and evolution of hydrothermal-vent fauna in the eastern Pacific Ocean. Proc. R. Soc. Lond. B 233: 347–366
Van Dover, C. L. (1990). Biogeography of hydrothermal vent communities along seafloor spreading centers. Trends Ecol. & Evol. 5: 242–246
Van Dover, C. L., Hessler, R. R. (1990). Spatial variation in faunal composition of hydrothermal vent communities on the East Pacific Rise and Galapagos Spreading Center. In: McMurray, G. R. (ed.) Gorda Ridge: a seafloor spreading center in the United States Economic Zone. Springer-Verlag, New York, p. 253–264
Weir, B. S., Cockerham, C. C. (1984). Estimating F-statistics for the analysis of population structure. Evolution, Lawrence, Kansas 38: 1358–1370
Wolf, P. G., Soltis, P. S. (1992). Estimates of gene flow among populations, geographic races, and species in the Ipomopsis aggregata complex. Genetics 130: 639–647
Wright, S. (1951). The genetical structure of populations. Ann Eugen. 15: 323–354
Wright, S. (1965). The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution, Lawrence, Kansas 19: 395–420
Wright, S. (1978). Evolution and genetics of populations. University of Chicago Press, Chicago
Zouros, E., Foltz, D. W. (1984). Possible explanations of heterozygote deficiency in bivalve molluscs. Malacologia 25: 583–591
Author information
Authors and Affiliations
Additional information
Communicated by J. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
France, S.C., Hessler, R.R. & Vrijenhoek, R.C. Genetic differentiation between spatially-disjunct populations of the deep-sea, hydrothermal vent-endemic amphipod Ventiella sulfuris . Marine Biology 114, 551–559 (1992). https://doi.org/10.1007/BF00357252
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00357252