Abstract
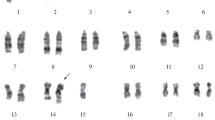
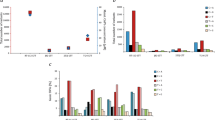
By multistep selection a set of clones and sublines possessing different levels of resistance to colchicine or adriablastin was obtained from the SV40-transformed Djungarian hamster cell lines, DM-15 and DMcap. Resistance to both colchicine and adriablastin is associated with an alteration of plasma membrane permeability leading to a decreased uptake of various drugs (3H-colchicine, 3H-cytochalasin B, 3H-actinomycin D, 3H-puromycin, 3H-vinblastine, 14C-chloramphenicol). The DNA of cells highly resistant to cholchicine can transmit resistance only to low dosages of the drug. Comparison of DNAs from wild-type and resistant cells digested by restriction endonucleases revealed new classes of repeated DNA sequences in resistant cell lines. The degree of DNA repetition was correlated with the level of drug resistance. The repeated DNA sequences evidently represent parts of the genome that are amplified in resistant cells. The size of the amplified sequences is 200–250 kilobase pairs (kb). Cell lines highly resistant to colchicine contain amplified DNA, which like mitochondrial DNA replicate asynchronously with the main portion of the cellular DNA and related but not identical DNA sequences are amplified in independent cell lines selected for resistance to colchicine, adriablastin, and actinomycin D. These cell lines display similar patterns of alterations of plasma membrane permeability. The amplified DNA sequences may contain a gene or genes the overexpression of which leads to change in plasma membrane permeability and a development of resistance to various drugs.
Similar content being viewed by others
References
Alitalo K, Schwab M, Lin CC, Varmus HE, Bishop JM (1983) Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in colon carcinoma. Proc Natl Acad Sci USA 80:1707–1711
Alt FW, Kellems RE, Bertino JR, Schimke RT (1978) Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem 253:1357–1370
Andrulis IL, Siminovitsch L (1982) Amplification of the gene for asparagine synthetase. In: Schimke RT (ed) Gene amplification. New York, Cold Spring Harbor Press, pp 75–80
Bahr G, Gilbert F, Balaban G, Engler W (1983) Homogeneously staining regions and double minutes in a human cell line: chromatin organization and DNA content. J Natl Cancer Inst 71:657–661
Baskin F, Rosenberg RN, Dev V (1981) Correlation of double minute chromosomes with unstable multidrug cross-resistance in uptake mutants of neuroblastoma cells. Proc Natl Acad Sci USA 78:3654–3658
Beach LR, Palmiter RD (1981) Amplification of the metallothionein-I gene in cadmium resistant cells. Proc Natl Acad Sci USA 78:2110–2114
Bech-Hansen NT, Till JE, Ling V (1976) Pleiotropic phenotype of colchicine-resistant CHO cells: cross-resistance and collateral sensitivity. J Cell Physiol 88:23–32
Bernardi G (1965) Chromatography of nucleic acids on hydroxyapatite. Nature 206:779–783
Bostock CL, Clark EM (1980) Satellite DNA in large marker chromosomes of methotrexate-resistant mouse cells. Cell 19:709–715
Bostock CL, Tyler-Smith C (1982) Amplification of dihydrofolate reductase genes and other DNA sequences in mouse cells. In: Schimke RT ed. Gene amplification. New York, Cold Spring Harbor Press, pp 15–22
Brennand J, Chinault AC, Konecki DS, Melton DW, Caskey CT (1982) Cloned cDNA sequences of the hypoxantine/guanine phosphoribosyltransferase gene from a mouse neuroblastoma cell line found to have amplified genomic sequences. Proc Natl Acad Sci USA 79:1950–1954
Dalla-Favera R, Wong-Staal F, Gallo RC (1982) One gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature 299:61–63
Danö K (1972) Cross resistance between Vinca alkaloids and anthracyclines in Ehrlich ascites tumor in vivo. Cancer Chemother Rep 56:701–708
Grund SH, Patil SR, Shah HO, Pauw PG, Stadler JK (1983) Correlation of unstable multidrug cross-resistance in Chinese hamster ovary cells with a homogeneously staining region on chromosome 1. Mol Cell Biol 3:1634–1647
Gudkov AV, Kopnin BP (1983) Analysis of amplified DNA sequences by restriction endonuclease digestion. Genetika 19:1045–1053 (In Russian)
Gudkov AV, Obukh IB, Serov SM, Naroditsky BS (1981) Variety of endogeneous proviruses in the genomes of chickens of different breeds. J Gen Virol 57:85–94
Hirt B (1967) Selective extraction of Polyoma DNA from infected mouse cell cultures. J Mol Biol 26:365–369
Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455:152–162
Kakpakova ES, Massino JS, Moiseenko EV (1976) Resistant to 6-mercaptopurine Djungarian hamster cell line. Genetika 12:56–61 (In Russian)
Kakpakova ES, Massino JS, Malachova EM (1981) Resistant to actinomycin D and 6-mercaptopurine (HPRT−) Djungarian hamster cell lines: karyotype, morphology, tumorogenicity. Genetika 17:460–467
Kanda N, Schreck R, Alt F, Bruns G, Baltimore D, Latt S (1983) Isolation of amplified DNA sequences from 1MR-32 human neuroblastoma cells: facilitation by fluorescence-activating flow sorting of metaphase chromosomes. Proc Natl Acad Sci USA 80:4069–4073
Kopnin BP (1981) Specific karyotypic alterations in colchicineresistant cells. Cytogenet Cell Genet 30:11–14
Kopnin BP, Massino JS, Gudkov AV (1985) Regular pattern of karyotypic alterations accompanying gene amplification in Djungarian hamster cells: Study of colchicine, adriablastin, and methotrexate resistance. Chromosoma 92:25–36
Melton DW, Brennand J, Ledbetter DH, Konecki DS, Chinault AC, Caskey CT (1982) Phenotypic reversion at the hprt locus as a consequence of gene amplification. In: Schimke RT (ed). Gene amplification. Cold Spring Harbor Press New York pp 59–66
Meyers MB, Biedler JL (1981) Increased synthesis of a low molecular weight protein in vincristine resistant cells. Biochem Biophys Res Comm 99:228–235
Milbrandt JD, Heintz NH, White WC, Rothman SM, Hamlin JL (1981) Methotrexate resistant Chinese hamster ovary cells have amplified a 135-kilobase-pair region that includes the dihydrofolate reductase gene. Proc Natl Acad Sci USA 78:6043–6047
Nunberg JH, Kaufman RJ, Schimke RT, Urlaub G, Chasin LA (1978) Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate resistant Chinese hamster ovary cell line. Proc Natl Acad Sci USA 75:5553–5556
Peterson RHF, Biedler JL (1978) Plasma membrane proteins and glycoproteins from Chinese hamster cells sensitive and resistant to actinomycin D. J Supramol Struct 9:289–298
Peterson RHF, Meyers MB, Spengler BA, Biedler JL (1983) Alteration of plasma membrane glycopeptides and gangliosides of Chinese hamster cells accompanying development of resistance to daunorubicin and vincristine. Cancer Res 43:222–228
Polotskaya AV, Gudkov AV, Kopnin BP (1983) Overproduction of specific polypeptide in Djungarian hamster and mouse cells resistant to colchicine and adriablastin. Bull Exp Biol Med 9:95–96 (In Russian)
Roberts JM, Axel R (1982) Gene amplification and gene correction in somatic cells. Cell 29:109–119
Roninson IB, Abelson HT, Housman DE, Nowell N, Varshavsky A (1984) Amplification of specific DNA segments correlates with multi-drug resistance in Chinese hamster cells. Nature 309:626–628
Schimke RT, Brown PC, Kaufman RJ, McGrogan M, Slate DL (1981) Chromosomal and extrachromosomal localization of amplified dihydrofolate reductase genes in cultured mammalian cells. Cold Spring Harbor Symp Quant Biol 45:785–796
Schwab M, Alitalo K, Varmus HE, Bishop JM, George D (1983a) A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumor cells. Nature 303:497–501
Schwab M, Alit K, Klempnauer K-H, Varmus HE, Bishop JM, Gilbert F, Brodeur G, Goldstein M, Trent G (1983b) Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and neuroblastoma tumour. Nature 305:245–248
Skovsgaard T (1977) Transport and binding of daunorubicin, adriamycin and rubidazone in Ehrlich ascites tumor cells. Biochem Pharmacol 26:215–222
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Stark GR, Brison O, Ardeshir F, Zieg J (1982) A general method for cloning amplified DNA: Properties of cloned amplified fragments near the CAD gene. In: Schimke RT (ed). Gene amplification New York, Cold Spring Harbor Press, pp 177–184
Stavrovskaya AA, Stromskaya TP, Serpinskaya AS, Kaszas I, Schuler D, Pogosianz HE (1976) Cross-resistance of transformed mouse cells to some drugs. Acta Biol Acad Sci Hung 27:37–44
Stromskaya TP, Kopnin BP, Stavrovskaya AA (1980) Clones of mouse near-diploid cells — a model for genetic analysis of traits of malignant phenotype. Vopr Oncol 26:50–55 (In Russian)
Wahl GM, Padgett RA, Stark GR (1979) Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphoacetyl)-L-aspartate-resistant hamster cells. J Biol Chem 254:8679–8687
Wahl GM, Allen V, Delbrück S, Eckhart W, Meinkoth J, Padgett R, de Saint Vincent BR, Rubnitz J, Stark G, Vitto L (1982) Analysis of CAD gene amplification using molecular cloning, gene transfer and cytogenetics. In: Schimke RT (ed) Gene amplification, Cold Spring Harbor Press, New York, pp 167–176
Wigler M, Pellicer A, Silverstein S, Axel GR, Urlaub G, Chasin LA (1979) DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci USA 76:1373–1377
Wigler M, Perucho M, Kurtz D, Dana S, Pellicer A, Axel GR, Silverstein S (1980) Transformation of mammalian cells with an amplifiable dominant acting gene. Proc Natl Acad Sci USA 77:3567–3571
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gudkov, A.V., Massino, J.S., Chernova, O.B. et al. Gene amplification in Djungarian hamster cell lines possessing decreased plasma membrane permeability for colchicine and some other drugs. Chromosoma 92, 16–24 (1985). https://doi.org/10.1007/BF00327241
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00327241